Translate this page into:
Serous surface papillary borderline tumor of ovary – An underdiagnosed entity
*Corresponding author: Ananya Panda, Department of Radiology, University of Iowa, Iowa City, United States. drananyapanda@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kaushik A, Panda A, Verma V, Nalwa A, Ghuman NK. Serous surface papillary borderline tumor of ovary – An underdiagnosed entity. Case Rep Clin Radiol. doi: 10.25259/CRCR_182_2023
Abstract
A young female presented with complaints of dysmenorrhea for 8 months. Ultrasound of the pelvis showed an echogenic mass in the left adnexa with papillary projections and internal vascularity and encasing the left ovary. Subsequent magnetic resonance imaging (MRI) showed an intensely enhancing, lobulated solid mass with a frond-like appearance, and papillary projections surrounding the left ovary reminiscent of a “sea-anemone.” The right ovary showed a cystic lesion without enhancing papillary projections or mural nodules. There was mild pelvic ascites and few peritoneal nodules in the pelvis. The presumptive radiologic diagnosis was serous surface papillary borderline tumor (SSPBT) of the left ovary and a benign cyst in the right ovary. The patient was treated with the left salpingo-oophorectomy, right ovarian cystectomy, peritoneal, and omental biopsies. Histopathology confirmed the diagnosis of SSPBT of the left ovary with microinvasion (<5 mm) of the ovarian surface. The right ovarian cyst was a follicular cyst. The peritoneal biopsies were positive for implants from SSPBT. This report highlights the diagnostic utility of “sea-anemone” sign and reviews the clinical and radiopathologic features of SSPBT.
Keywords
Serous surface papillary borderline tumor
Ovary
Magnetic resonance imaging
Ultrasound
Borderline tumor
INTRODUCTION
Serous epithelial tumors of ovary range from benign, borderline and malignant subtypes, based on the degree of biologic aggressiveness. Within borderline ovarian tumors, serous surface papillary tumors represent a specific subtype, with a distinct radiologic appearance. Familiarity with this entity can improve pre-operative imaging diagnosis and inform patient management.
CASE REPORT
A 28-year-old unmarried female with no previous comorbidities presented with complaints of dysmenorrhea for 8 months. The patient attained menarche at 13 years, with regular cycles every 30 days, lasting 4 days with average menstrual flow. There was no other comorbidity. Per abdominal examination was unremarkable. She was prescribed oral contraceptive pills for dysmenorrhea and subsequently underwent imaging evaluation with ultrasound and magnetic resonance imaging (MRI) of the pelvis.
Ultrasound showed a lobulated, heterogeneously echogenic, left adnexal mass with solid-microcystic appearance and irregular papillary projections measuring 10 cm in largest dimensions and completely surrounding the left ovary. The left ovary itself showed preserved follicular morphology. On color Doppler, there was moderate color flow within the mass (color score 3) [Figure 1]. On the right side, there was a 6 cm anechoic cystic lesion without internal septa, nodules, or vascularity. There was mild pelvic ascites. The left adnexal mass was categorized as Ovarian-Adnexal Reporting and Data System (O-RADS) US 5 lesion (risk of malignancy >50%) and the right ovary mass was categorized as O-RADS US 2 lesion (almost certainly benign).[1]

- (a and b) Transabdominal ultrasound shows heterogeneous echogenic solid mass in the left adnexal region (solid white arrow in a) encasing the left ovary (solid black arrow in b). (c and d) Color Doppler images show moderate color flow inside the mass (dashed white arrow in d).
Subsequent contrast-enhanced MRI revealed a 10 cm × 9.6 cm × 6 cm size, T2-weighted (T2W) hyperintense lobulated solid mass with papillary projections, and frond-like appearance entirely surrounding the left ovary suggestive of “sea-anemone” sign. The mass showed mild diffusion restriction and intense enhancement. There was no hemorrhage or necrosis within the mass. Left ovary was seen separately within the mass with distorted surface contour but intact follicles [Figure 2]. There was no obvious ovarian invasion on MRI. The right ovary was replaced by a 6.3 cm × 4.5 cm × 4.3 cm size, thin-walled biloculated cystic lesion without solid enhancing components, hemorrhage, or diffusion restriction [Figure 2]. There were mild ascites and few indeterminate peritoneal nodules in the pelvis. Uterus and cervix were normal. There was no pelvic lymphadenopathy. In view of an enhancing mass with papillary morphology encasing the left ovary and peritoneal nodules, this was categorized as O-RADS MRI 5 lesion with a presumptive radiologic diagnosis of serous surface papillary borderline tumor (SSPBT). The biloculated cystic lesion in the right ovary without solid or enhancing components was categorized as O-RAD MRI 2 lesion.[2]
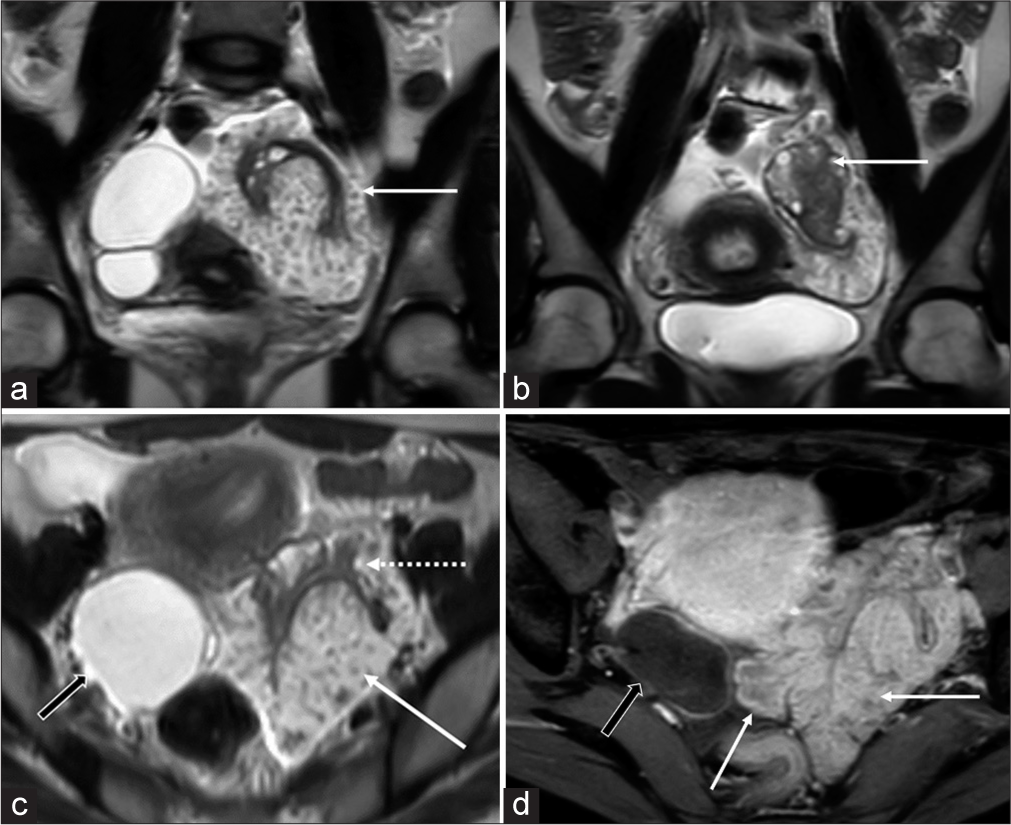
- (a and b) Coronal T2-weighted (T2w) images show lobulated hyperintense mass with papillary projections (solid white arrow in a) encasing the left ovary (solid white arrow in b). The follicular morphology of the left ovary is preserved while being surrounded by the mass on its surface. (c) Axial T2w image shows the surface papillary morphology of the tumor (solid white arrow), relative to the left ovary (dotted white arrow), and incidental right ovarian cyst (black arrow). (d) Axial post-contrast T1-weighted image shows moderate enhancement of the mass (relative to the uterus) with frond-like projections (solid white arrows) and non-enhancing right ovarian cyst (black arrow).
Laboratory investigations showed elevated serum CA-125 level-527 U/mL (normal < 35U/mL) and mildly increased inhibin 103 pg/mL (normal <80 pg/mL). Serum lactate dehydrogenase, serum alpha-fetoprotein, and serum carcinoembryonic antigen were within normal limits. The patient was taken for elective staging laparotomy. Intraoperative findings revealed a large fungating, polypoidal mass in the left adnexa and a cystic lesion in the right ovary. The patient underwent left salpingo-oopherectomy, right ovarian cystectomy, and peritoneal and omental biopsies.
Gross pathology showed a frond-like mass encasing the left ovary [Figure 3]. On histopathology, the left-oophorectomy specimen confirmed the diagnosis of serous surface papillary borderline tumor showing cystic tissue and tumor cells arranged in micropapillae, papillae, and fused cribriform patterns [Figure 4]. A small focus of micro-invasion (<5 mm) was noted in the left ovary stroma. Right ovarian cystectomy specimen was diagnosed as a follicular cyst on pathology. Sections from peritoneal biopsy showed a focal tumor implant floating in it. Sections from omentum were negative for deposits or malignancy in the sections. The post-operative period was uneventful and the patient was discharged on day 6. Follow-up at 2 months was uneventful. No further chemotherapy was considered in this patient.
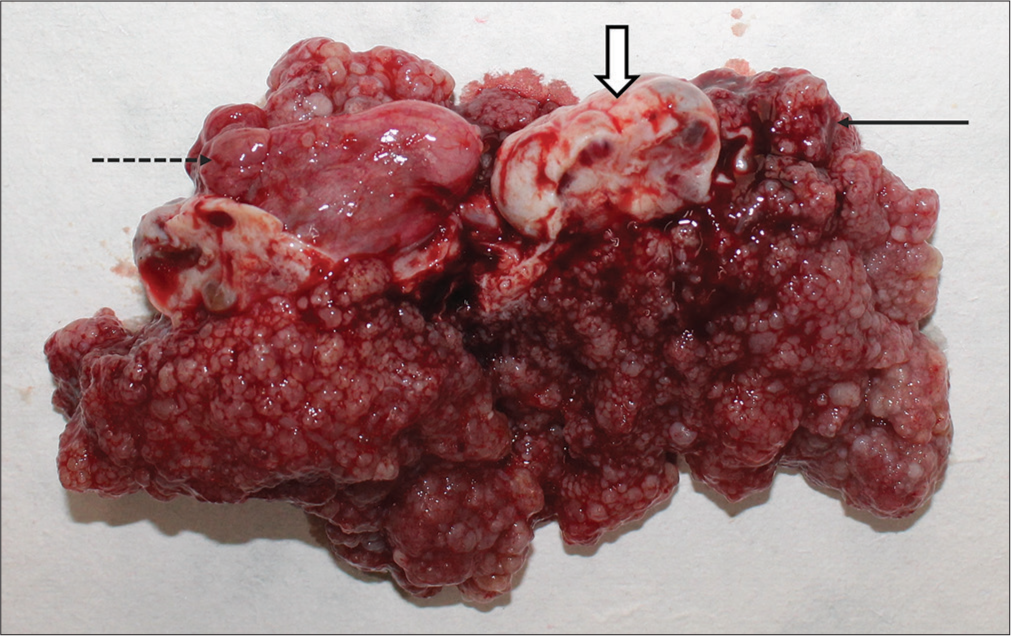
- Gross specimen showing frond like appearance of the mass (black arrow) encasing the left ovary (white block arrow) and right ovarian cyst (dotted black arrow).
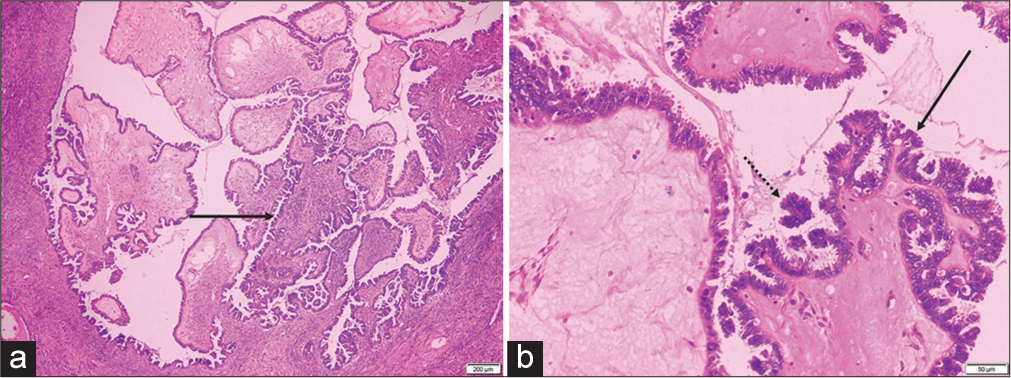
- (a) Hematoxylin & Eosin × 20 image shows architecturally complex papillae with hierarchical internal branching (solid black arrow). (b) Hematoxylin & Eosin × 100 image shows papillae lined by stratified epithelium (solid black arrow) with tufting and cell detachment (dotted black arrow).
DISCUSSION
According to their biologic aggressiveness, the serous subtype of ovarian epithelial tumors are classified as benign (60%), borderline (15%), and malignant (25%). According to the degree of macroscopic proliferation, serous epithelial ovarian tumors can also be classified as cyst-forming, surface proliferative, or stromal proliferative. Accordingly, there are three types of serous tumors of the ovary: serous cystic tumors, SSPBT, and serous cystadenofibromas.[3] SSPBTs are a distinct subtype of serous tumors of the ovary, in which the tumor is confined to the ovarian surface, and the surface and morphology of the ovary are preserved. SSPBTs are uncommon entities with very few studies describing their sonographic and MRI appearances. These tumors characteristically display a surface proliferative pattern and papillary excrescences encasing the normal appearing ovary.[3] On ultrasound, SSPBTs appear as echogenic solid masses with irregular, nodular, or papillary margins with an internal branching appearance. Inside these masses, normal-appearing ovaries containing multiple follicles can be clearly discriminated, although a mild degree of distortion of the normal ovary can be present.[4] On MRI, these tumors show a complex solid-cystic appearance with intermediate signal intensity on both T1W- and T2W images. Macroscopically, the surface stroma frequently branches into exophytic papillary stalks with exuberant frond-like excrescences, mimicking the likeness of a “sea anemone” also described as the “sea-anemone” sign on MRI.[5] After contrast enhancement, the tumors show enhancement similar to or greater than the ovary, but the sharp demarcation from normal ovarian parenchyma is still maintained,[2] as also seen in our case.
Although these lesions are categorized as O-RADS 5 lesions due to the presence of solid components and peritoneal implants, it is important to recognize that these lesions have a much better prognosis compared to other borderline and malignant serous tumors of the ovary with peritoneal metastases. The most important finding that differentiates SSPBTs from other ovarian tumors is the presence of normal ovarian stroma or at least some ovarian stroma partially or completely surrounded by solid tumors. Of note, elevated serum CA-125 levels are seen in both SSPBTs and malignant serous ovarian tumors and cannot be used for differentiation.
While SSPBTs are borderline tumors, these can show microinvasion to the ovarian surface on pathology. SSPBTs may also be precursor to serous surface papillary carcinomas as part of the adenoma-carcinoma sequence. The earliest radiologic description of SSPBTs by Tanaka et al. reviewed six cases, all of which showed bilaterality and three cases showed extraovarian spread.[5] In our case too, a focus of peritoneal implant was present. Of note, extraovarian spread in SSPBT is referred to as implants instead of metastases that can either be invasive or non-invasive implants.[5] Despite extraovarian implants, SSPBTs have a better prognosis than other serous borderline tumors of the ovary.
The pathologic hallmark of SSPBT is papillary architecture and internal branching (PA&IB) pattern.[5] SSPBTs showing isolated PA&IB patterns are more commonly seen in younger patients and have favorable prognosis. However, the presence of micropapillary and cribriform patterns have a higher risk of conversion into serous surface papillary carcinoma.[5] Due to the younger age of onset, earlier stage at presentation, and better prognosis, patients can undergo conservative surgery with unilateral salpingooopherectomy to preserve fertility.[5,6] The role of adjuvant therapy in SSPBTs is not well-established. In one study by Park et al., two out of five patients received adjuvant chemotherapy and five patients were disease-free after 2 years follow-up.[3]
DIFFERENTIAL DIAGNOSIS
The differential diagnosis for this case is mentioned in Table 1 under Differential Diagnosis.
| Sr. No. | Differential diagnosis | Points in this case suggesting diagnosis | Points in this case against diagnosis |
|---|---|---|---|
| 1. | High-grade serous ovarian malignancy |
|
|
| 2. | Granulosa cell tumor |
|
|
| 3. | Pelvic tuberculosis/Pelvic inflammatory disease |
|
|
O-RADS: Ovarian Adenxal Reporting and Data System, CA-125: Cancer Antigen-125
CONCLUSION
SSPBTs of the ovary are a distinct subtype of ovarian malignancy with a characteristic radiologic appearance and a favorable prognosis despite extraovarian disease.
TEACHING POINTS
Serous surface papillary borderline tumor of the ovary is a recently described clinical entity with characteristic imaging appearances, forming ‘sea-anemone” like masses encasing the surface of ovary.
Pre-operative recognition of this entity is helpful as SSPBT has a better prognosis compared to other serous borderline and malignant tumors of ovary despite extraovarian disease, enabling patients to be offered less-radical surgeries.
MCQs
-
Which serum tumor marker is most commonly elevated in epithelial neoplasms of ovary?
CA-19.9
CA-125
LDH
AFP
Answer Key: b
-
What best describes the imaging findings in serous surface papillary borderline tumors of the ovary?
Papillary projections involving surface of the ovary with partial or complete ovarian stroma uninvolved
Large solid cystic ovarian mass with internal papillary projections
Predominantly solid mass with internal areas of fat and calcifications
None of the above
Answer Key: a
-
Which statement is not true?
SSPBTs are more common in younger age group
SSPBTs appear as sea-anemone like masses
Peritoneal implants in SSPBTs demonstrate poor prognosis
SSPBTs have a better prognosis than other serous borderline tumors of the ovary
Answer Key: c
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- O-RADS US risk stratification and management system: A consensus guideline from the ACR ovarian-adnexal reporting and data system committee. Radiology. 2020;294:168-85.
- [CrossRef] [PubMed] [Google Scholar]
- O-RADS MRI risk stratification system: Guide for assessing adnexal lesions from the ACR O-RADS committee. Radiology. 2022;303:35-47.
- [CrossRef] [PubMed] [Google Scholar]
- Ovarian serous surface papillary borderline tumor: Characteristic imaging features with clinicopathological correlation. Br J Radiol. 2018;91:20170689.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound diagnosis of serous surface papillary borderline ovarian tumor: A case series with a review of the literature. J Clin Ultrasound. 2015;43:573-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ovarian serous surface papillary borderline tumors form sea anemone-like masses. J Magn Reson Imaging. 2011;33:633-40.
- [CrossRef] [PubMed] [Google Scholar]
- Serous borderline tumor with micropapillary pattern of the right ovary that developed 6 recurrences over 30 years after primary surgery. Gynecol Oncol Rep. 2018;25:45-7.
- [CrossRef] [PubMed] [Google Scholar]








