Translate this page into:
Unusual aggressive central nervous system involvement in CLIPPERS syndrome
*Corresponding author: Osman Kahraman, Department of Radiology, Medipol Mega University Hospital, Istanbul, Turkey. osman.kahraman@medipol.edu.tr
-
Received: ,
Accepted: ,
How to cite this article: Kahraman O, Ermiş A, Karaalioğlu B. Unusual aggressive central nervous system involvement in CLIPPERS syndrome. Case Rep Clin Radiol. doi: 10.25259/CRCR_196_2023
Abstract
Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids known as CLIPPERS syndrome is a rare and recently described inflammatory disorder of the central nervous system. Ataxia and cognitive dysfunction are usual symptoms and are dramatically responsive to steroid treatment. We present a case of a 58-year-old female patient, clinically diagnosed CLIPPERS syndrome with unusual aggressive involvement of supratentorial white matter and spinal cord that showed complete recovery with significant atrophy after steroid therapy.
Keywords
CLIPPERS
Supratentorial
Pons
Atrophy
Steroid
INTRODUCTION
Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids known as CLIPPERS syndrome is a rare and recently described inflammatory disorder of the central nervous system.[1] It predominantly affects the pons and cerebellum. Ataxia, cognitive dysfunction, and myelopathy with subacute and progressive courses are usual presenting symptoms.[2] Proper diagnosis with suspicious clinical symptoms and characteristic radiological findings is feasible, which is also verified with a dramatic steroid response.[3] To date, approximately 100 cases have been reported majorly with typical radiological findings.[4] We present a case clinically diagnosed CLIPPERS syndrome with unusual aggressive involvement of supratentorial white matter and spinal cord that showed complete recovery with significant atrophy after steroid therapy.
CASE REPORT
A 58-year-old female patient was admitted with 2-week history of walking inability. She had progressive muscle weakness and gait imbalance complaints for 3 months with accompanying visual hallucinations, mood, and personality changes for past few weeks. The patient does not have a history of smoking and alcohol use, and there is no history of hereditary disease in her family.
Neurological examination showed dysphasia, severe ataxia, hyperactive deep tendon reflexes, bilateral clonus, and Babinski positivity. She was unable to stand and had 4/5 left upper and bilateral lower extremity muscle weakness, but the superficial sensation was normal. Electromyography and electroencephalography results were normal. Mini–mental state examination score was 26/30. Routine biochemical examination, liver and kidney function, complete blood count and coagulation parameters, and serum vitamin levels (A, B1, and E) were within normal references. Hepatitis B and C, human immunodeficiency virus, venereal disease research laboratory, and tuberculosis polymerase chain reaction (PCR) were negative. Autoimmune disease panel tests – ANA, anti-dsDNA, lupus anticoagulants, anti-ENA (SCL-70, SS-a, Sm), ANCA, anti-NMO, and anti-MOG – were normal. Serum paraneoplastic syndrome tests and autoimmune encephalitis panel were negative. In cerebrospinal fluid (CSF) analysis, glucose level was normal, 3 lymphocytes and high protein (84 mg/dL) were revealed. Neither oligoclonal bands nor atypical cells were observed. CSF culture and CSF PCR panel of meningitis-encephalitis were negative.
Magnetic resonance imaging (MRI) showed widespread patchy, punctate, and curvilinear nodular contrast enhancement, prominent at the posterior frontal, parietal, and occipital white matter with lesser degree pons and cerebellar white matter involvement. Accompanying confluent and widespread fluid-attenuated inversion recovery hyperintensity with increased diffusion throughout supratentorial and pontocerebellar white matter around the lesions was seen, representing marked white matter edema. Extensive nodular enhancement similar to cerebral lesions was observed all through the spinal cord with coexisting transverse myelitis-like cord edema and extension [Figure 1]. Although unusual, a widespread presentation of CLIPPERS syndrome was considered in diagnosis with characteristic features. Vasculitis, central nervous system infectious diseases, neoplastic, paraneoplastic diseases, and other systemic autoimmune inflammatory diseases existing in differential diagnosis were excluded with laboratory tests.
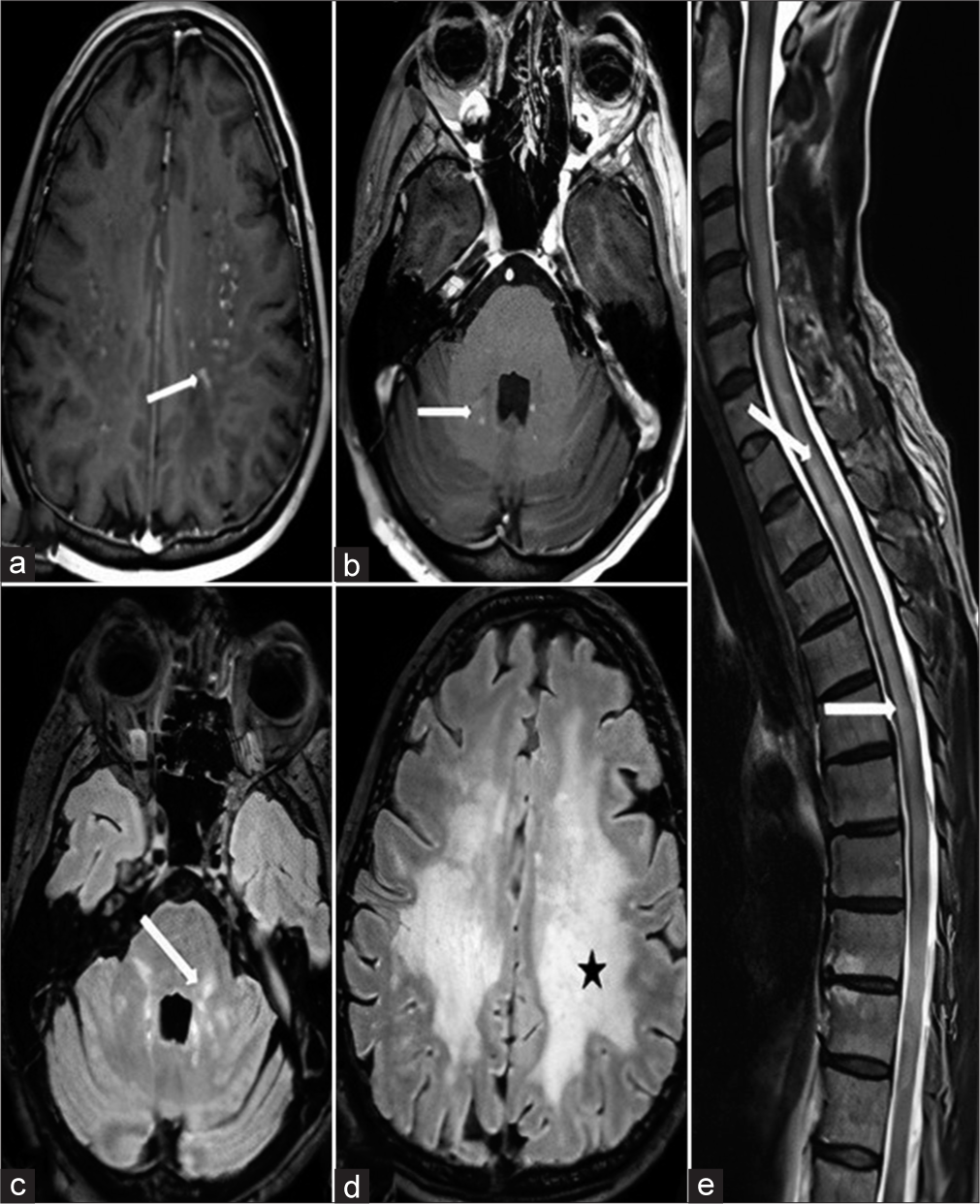
- A 58-year-old female with chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroid syndrome, first magnetic resonance images. (a) Axial post-contrast T1-weighted section revealed tiny nodular and curvilinear contrasted lesion (white arrow) in centrum semiovale region. (b) Axial post-contrast T1-weighted section revealed tiny nodular and curvilinear contrasted lesion (white arrow) in right ponto-cerebellar region. (c and d) Axial FLAIR sections revealed perilesional white matter edema in left ponto-cerebellar (white arrow) and centrum semiovale (black star) regions, respectively. (e) Sagittal T2-weighted section revealed extensive spinal cord edema in cervical and thoracic regions (white arrows).
1000 mg pulse corticosteroid was given for 7 days, followed by 32 mg/day oral methylprednisolone. At the 1st month, the neurological examination of the patient revealed that her cognitive functions returned completely to normal but she had mild (+4/5) lower extremity proximal flexor muscle weakness and clumsiness in tandem walking. MRI imaging at the end of 1st month with continuing steroid treatment showed a prominent reversal of contrast-enhanced white matter lesions with few residue supratentorial punctate lesions. Transverse myelitis-like diffuse cord edema and contrasted cord lesions showed almost complete recovery with solitary C6 and T1 level sub-centimetric nodular enhancements [Figure 2]. 7th-month follow-up MRI showed a complete reversal of contrasted white matter lesions of neural parenchyma, with sequela gliosis of supratentorial white matter and central spinal cord with accompanying cerebral atrophy [Figure 3]. Oral methylprednisolone treatment was reduced to 24 mg/day and planned to end gradually.
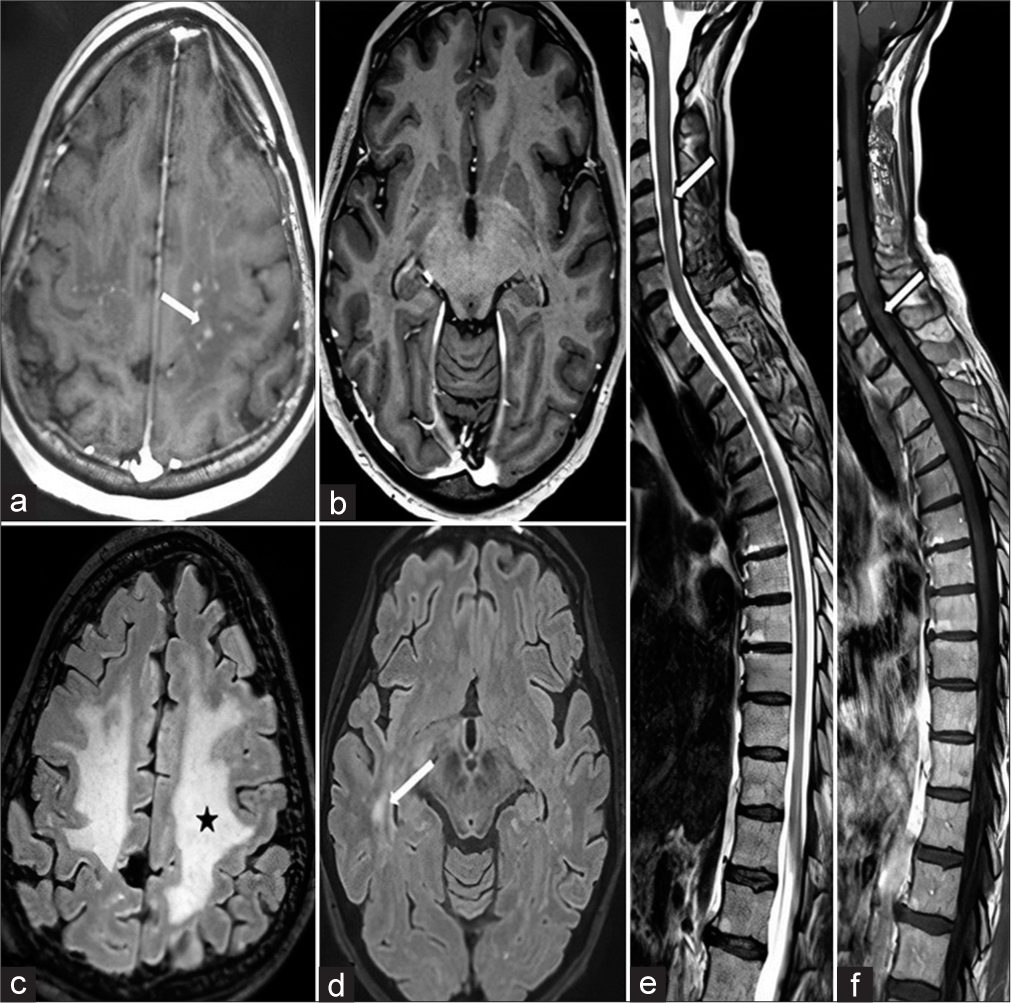
- Magnetic resonance imaging taken 1 month after starting the treatment. (a) Axial post-contrast T1 weighted section revealed a few residual nodular contrast enhanced white matter lesions seen in centrum semiovale region (white arrow). (b) Axial post-contrast T1 weighted section also revealed complete disappearence of contrasted lesions in temporo-occipital region. (c and d) Axial FLAIR sections revealed reduced vasogenic edema seen in centrum semiovale region (black star) and significant reversal of hyperintensities in temporo-occipital region (white arrow), respectively. (e) Sagittal T2 weighted section revealed edema almost completely regressed with a few residue signals (white arrow). (f) Sagittal post-contrast T1-weighted section revealed almostly disappearence of contrasted lesions (white arrow).
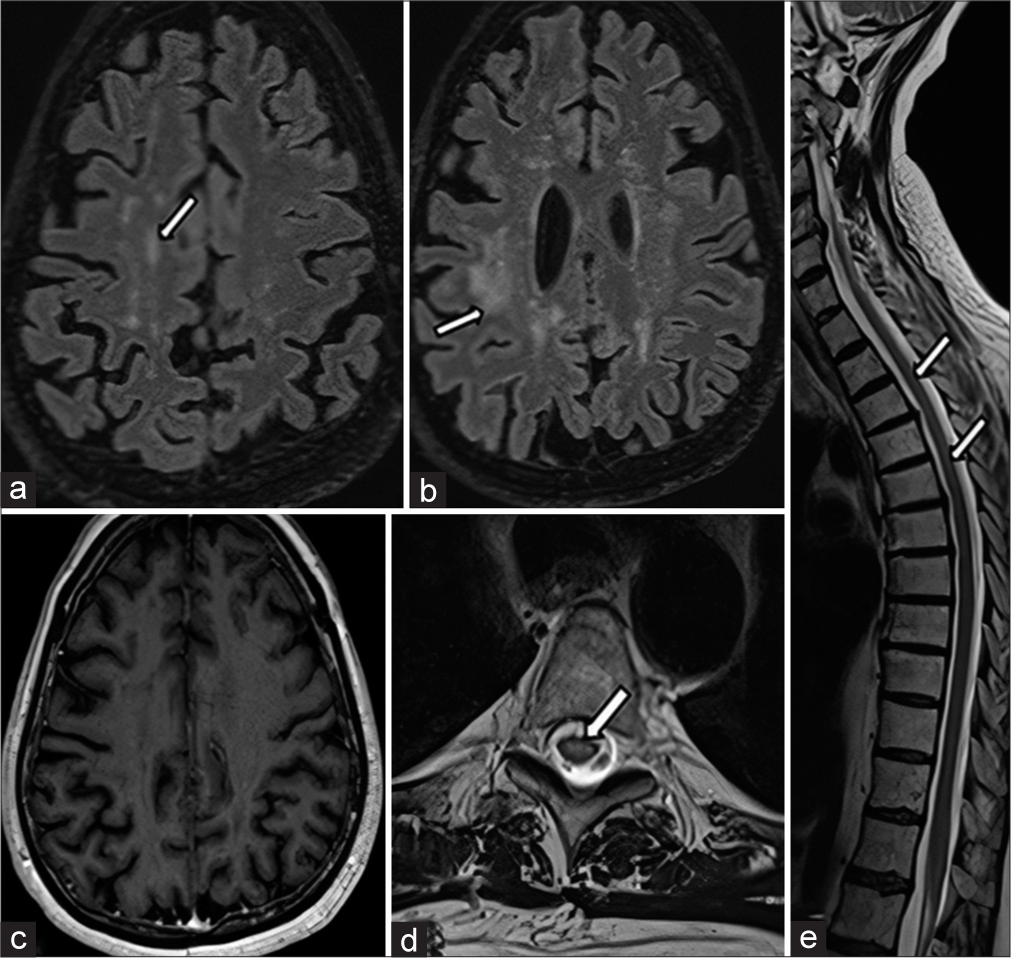
- Magnetic resonance imaging taken 7 months after starting the treatment. (a and b) Axial fluid-attenuated inversion recovery sections revealed mild residual sequel gliotic white matter intensities (white arrows) with accompanying prominent parenchymal atrophy (which can easily be distinguished when compared with initial images). (c) Axial post-contrast T1 weighted sections revealed a complete reversal of cerebral contrast-enhanced nodules seen in the centrum semiovale region. (d and e) Axial and sagittal T2-weighted sections revealed gliosis in the central regions with detectable cord atrophy at cervical and thoracic locations (white arrows), respectively.
DISCUSSION
CLIPPERS syndrome is a lymphocytic inflammatory disease of the central nervous system. It is characterized as inflammation with non-specific T lymphocytic infiltration in perivascular spaces of white matter with increased vulnerability to the brain stem, cerebellum, and spinal cord.[1] Since it was first identified, the diagnosis was made on the basis of clinical symptoms, radiological finding, and sometimes histopathology to exclude other possible differentials.[2,3,5] In our case, biopsy was not done, but all other possible mimickers were excluded from the laboratory [Table 1].[6,7]
| Diseases | Predominant Location | Magnetic Resonance Imaging Signs |
|---|---|---|
| CLIPPERS | Pons, cerebellum, and basal ganglia | Punctate or curvilinear gadolinium-enhancing lesions in pons |
| Glial fibrillary acidic protein astrocytopathy | Brain and spinal cord | Linear radial periventricular enhancement pattern |
| Neuro-Behçet’s disease | Brain | Non-specific multifocal lesions |
| Neurosarcoidosis | Brain | Nodular enhancing lesions and granuloma |
| Primary central nervous system lymphoma | Brain | Enhancing lesions with mass effect |
| Infections | Brain and spinal cord | Varied, may include multiple enhancing lesions |
| Vasculitis | Brain and spinal cord | Vasculitic changes, multifocal lesions, and infarcts |
| Paraneoplastic Syndromes | Brian and spinal cord | Non-specific multiple enhancing lesions |
CLIPPERS: Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids
In literature, there have been either supratentorial or spinal cord lesions in a significant proportion of presented cases with varying severity.[8-10] Pittock et al. reported spinal cord lesions in three of eight cases without supratentorial involvement and atrophy.[1] Tobin et al. described supratentorial lesions in 16 of 35 patients and spinal cord lesions in 11 of 35.[2] Although rare, it is known that atrophy may develop in the affected regions of the central nervous system in follow-ups.[6,8] In the literature, few cases reported parenchymal atrophy following treatment. Simon et al. published three cases out of five that developed parenchyma atrophy in the involved regions of supratentorial white matter. They also reported spinal cord involvement in one patient without atrophy.[3] Taieb et al. presented atrophy in one of four patients in the affected spinal cord.[6]
Widespread involvement of CLIPPERS syndrome was shown in 7T MRI studies.[11] In our case, where we took 3T MRI images, these widespread involvements are also present. As is well known in CLIPPERS, our case also showed dramatic radiological and clinical improvement with steroid therapy. As a result, we can say that in patients with aggressive central nervous system involvement of CLIPPERS syndrome, despite the excellent response to steroid treatment, permanent parenchymal damaged, and atrophy risk is relatively high. We think that to reveal the long-term consequences, the follow-up of these patients will make a significant contribution to the literature.
CONCLUSION
Characteristic radiological findings are not only important in the differential diagnosis of CLIPPERS syndrome, but also are very valuable for the follow-up of this disease and seeing its long-term results. A dramatic response to steroid treatment does not always indicate complete recovery of this disease. Although the possibility of leaving behind sequelae, atrophic brain tissue is rare, it should be kept in mind.
TEACHING POINTS
CLIPPERS syndrome is an inflammatory disorder of the central nervous system. It is characterized by punctate and curvilinear gadolinium enhancement on MRI in the pons and cerebellum and is dramatically responsive to steroid treatment
Rarely, aggressive central nervous system involvement of CLIPPERS syndrome, despite the excellent response to steroid treatment, permanent parenchymal damaged, and atrophy risk is relatively high.
MCQs
-
Which of the following MRI radiological findings is characteristic of CLIPPERS syndrome?
Periventricular finger-like hyperintense areas
Enlargement of the cerebral ventricles
Hippocampal atrophy
Punctate enhancement peppering the pons
Answer Key: d
-
Which of the following is a laboratory marker of CLIPPERS syndrome?
ANCA
Anti-dsDNA
None
Leukocytosis
Answer Key: c
-
Which of the following treatment modalities is used primarily in CLIPPERS disease?
Methotrexate
Anti-TNF agents
Corticosteroid (applies)
Antibiotics
Answer Key: c
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) Brain. 2010;133:2626-34.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic criteria for chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) Brain. 2017;140:2415-25.
- [CrossRef] [PubMed] [Google Scholar]
- Expanding the clinical, radiological and neuropathological phenotype of chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) J Neurol Neurosurg Psychiatry. 2012;83:15-22.
- [CrossRef] [PubMed] [Google Scholar]
- A case report of CLIPPERS syndrome and literature review. Medicine (United States). 2021;100:E26090.
- [CrossRef] [PubMed] [Google Scholar]
- Case 309: Autoimmune glial fibrillary acidic protein astrocytopathy. Radiology. 2023;306:293-8.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcomes of CLIPPERS (Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) in a consecutive series of 12 patients. Arch Neurol. 2012;69:847-55.
- [CrossRef] [PubMed] [Google Scholar]
- A distinct case of CLIPPERS with predominant spinal cord involvement (P3.419) Neurology. 2018;90(15 Supplement):p419.
- [CrossRef] [Google Scholar]
- CLIPPERS: Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. Review of an increasingly recognized entity within the spectrum of inflammatory central nervous system disorders. Clin Exp Immunol. 2014;175:385-96.
- [CrossRef] [PubMed] [Google Scholar]
- CLIPPERS with longitudinally extensive transverse myelitis: Role of T versus B cells. J Neurol Sci. 2018;385:96-8.
- [CrossRef] [PubMed] [Google Scholar]
- CLIPPERS and its mimics: Evaluation of new criteria for the diagnosis of CLIPPERS. J Neurol Neurosurg Psychiatry. 2019;90:1027-38.
- [CrossRef] [PubMed] [Google Scholar]
- Widespread inflammation in CLIPPERS syndrome indicated by autopsy and ultra-high-field 7T MRI. Neurol Neuroimmunol Neuroinflamm. 2016;3:e226.
- [CrossRef] [PubMed] [Google Scholar]








