Translate this page into:
Fighting shadows: Understanding a complex tumor
*Corresponding author: Seetharaman Cannane, Department of Radiology, Kovai Medical Center and Hospitals, Coimbatore, Tamil Nadu, India. drcseetharaman@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sugumar R, Rajasekaran S, Cannane S. Fighting shadows: Understanding a complex tumor. Case Rep Clin Radiol. doi: 10.25259/CRCR_60_2024
Abstract
Synovial sarcoma (SS), a complex soft-tissue cancer, commonly affects young individuals across various body areas. Despite its name, SS does not arise from synovial tissue, creating challenges in diagnosis and treatment. Advanced imaging like magnetic resonance imaging is crucial for accurate assessment. Understanding its diverse nature and complex imaging features is essential for managing this condition effectively. In this case report, we wish to highlight the features of SS in a 20-year-old male who presented with hoarseness of voice and dysphagia for 2 months.
Keywords
Synovial sarcoma
Synovium
Biphasic
INTRODUCTION
Synovial sarcoma (SS) is a malignant mesenchymal tumor, representing 5–10% of all primary soft-tissue malignancies, commonly affecting adolescents and young adults. SSs are rare in the head-and-neck region. Accurate diagnosis and staging, primarily through magnetic resonance imaging (MRI), are crucial for effective treatment and optimal patient outcomes.
CASE REPORT
A 20-year-old male presented with hoarseness of voice and dysphagia for 2 months. The initial work-up showed normal blood parameters. The patient was evaluated with contrast-enhanced computed tomography (CECT) of neck followed by MRI of neck.
Direct laryngoscopy (DL) showed intact pharyngeal and laryngeal mucosal lining. On CECT [Figure 1], well-defined lobulated solid cystic lesion was seen in the hypopharynx with epicenter just behind the left pyriform fossa. Mild heterogeneous enhancement of the solid component was noted in the post-contrast images. There was no evidence of erosion of the thyroid cartilage or any laryngeal cartilages. No features of invasion into adjacent structures were observed.
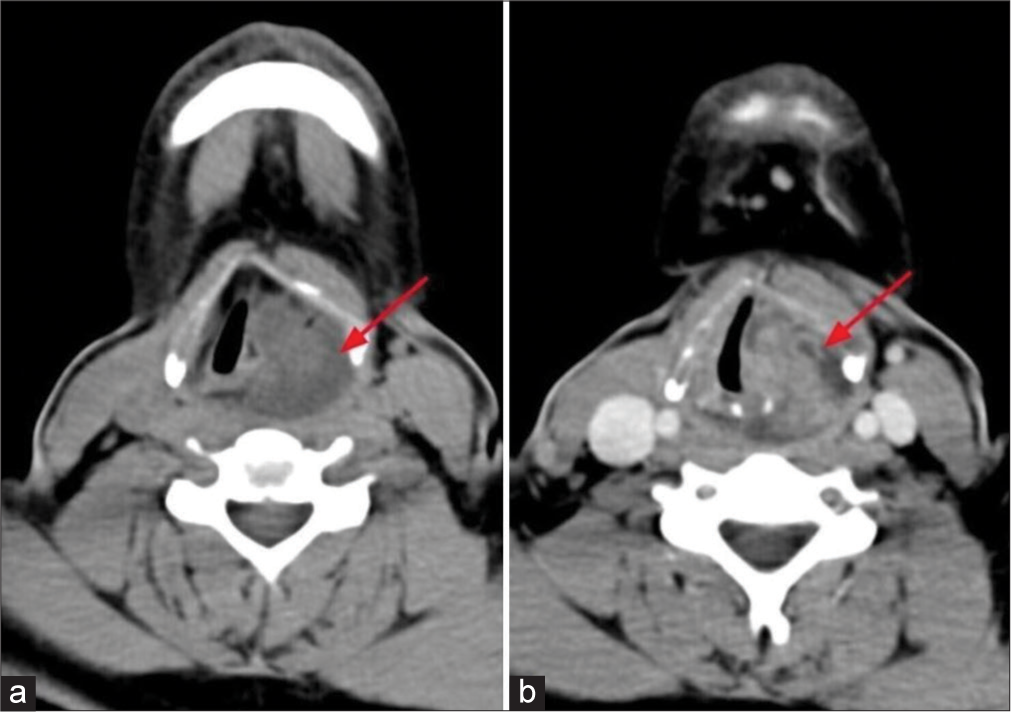
- Axial plain and contrast enhanced computed tomography of neck shows (a) well-defined lobulated predominantly hypodense lesion in hypopharnyx (red arrow). (b) Solid areas shows enhancement in venous phase (red arow).
On MRI [Figure 2], well-defined lobulated solid cystic lesion measuring about 5.1 × 3.0 × 2.8 cm [craniocaudal (CC) × anteroposterior (AP) × transverse (TR)] was noted in the hypopharynx with epicenter just behind the left pyriform fossa. Solid components appear isointense on T1 and hyperintense on T2 to skeletal muscle and the solid component showed significant diffusion restriction and heterogenous post contrast enhancement. No evidence of erosion of thyroid cartilage/any laryngeal cartilages seen. Anteriorly the lesion extends up to the inferior border of thyroid cartilage abutting the strap muscles. No definite evidence of extension or abnormal signal intensity in the visualized bones. Based on these imaging features, possibility of mesenchymal neoplasm (likely SS) was given and was evaluated further. Histopathological examination (HPE) [Figure 3] revealed features of SS.
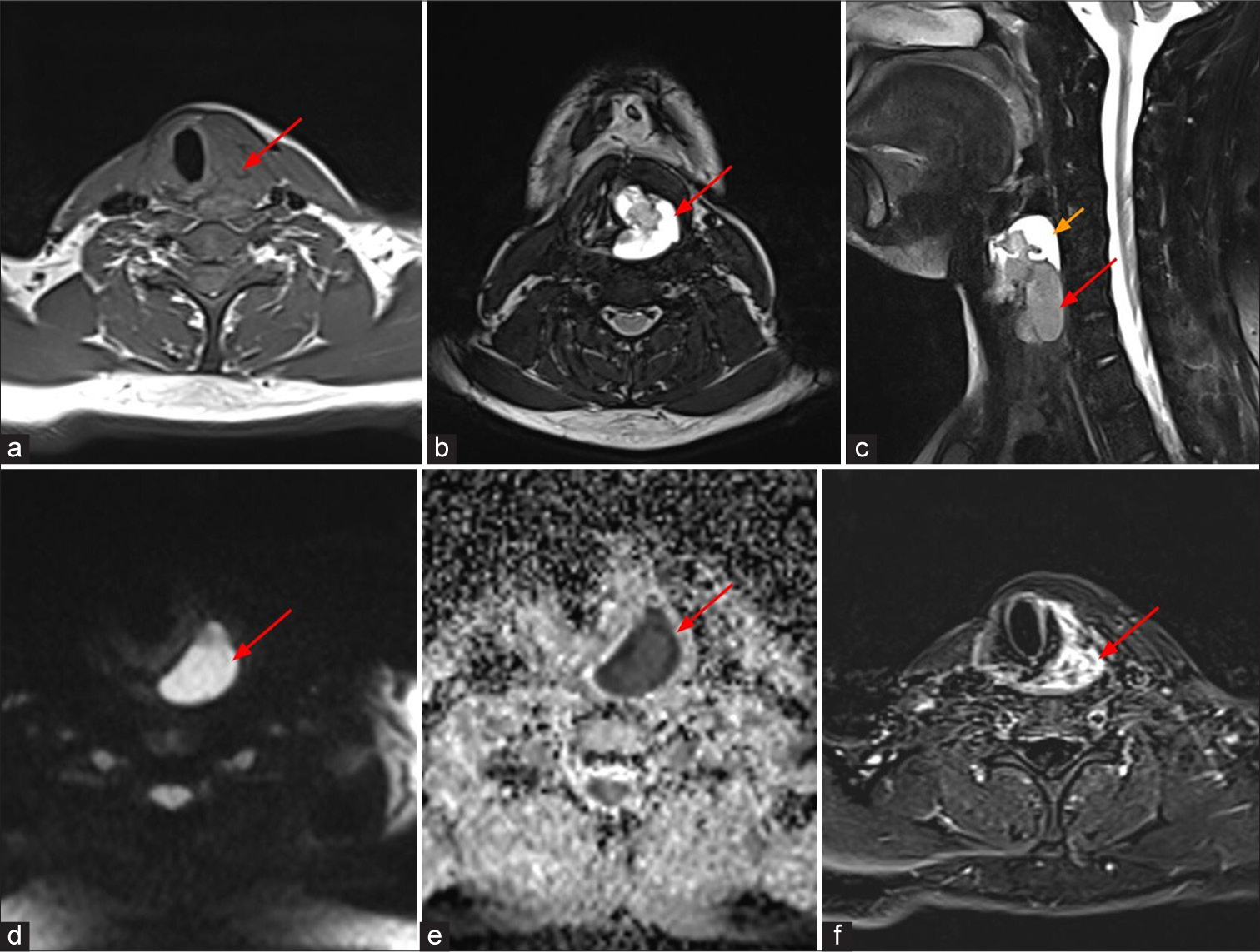
- A 20-year-old male who presented with hoarseness of voice and dysphagia, MRI shows well-defined lobulated solid cystic lesion in hypopharynx just behind the left pyriform fossa, which appears isointense to skeletal muscle (red arrow) on (a) axial T1 weighted image, hyperintense (red arrows) on (b) axial and (c) sagittal T2 weighted images. (c) Sagittal T2 weighted image shows T2 hyperintense cystic component (yellow arrow) on superior aspect of the lesion. The solid component shows restricted diffusion (red arrows) on (d) diffusion weighted sequence and (e) apparent diffusion coefficient and (f) heterogeneous enhancement (red arrow) on post contrast subtracted image.
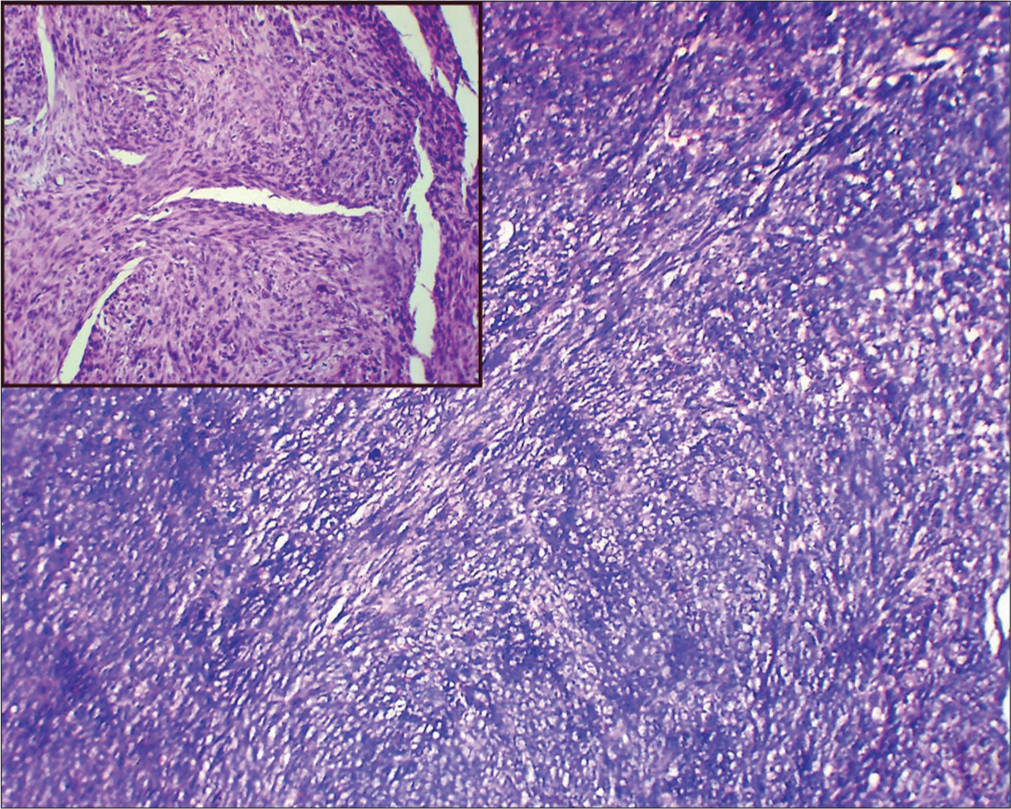
- Low-power and high-power (Inset image) histopathology image shows spindle cells arranged in fascicles. Stain: Hematoxylin and eosin.
DISCUSSION
SS of hypopharynx is a malignant mesenchymal neoplasm, and it is the third most common soft-tissue tumor account for approximately 5–10% of all primary soft-tissue malignancies.[1,2] It typically manifests in older children and young adults, with an average age of 32 years. Both men and women are equally affected by the condition. SS commonly presents in the lower extremities, particularly around the knee joint. Nevertheless, it has the propensity to arise across diverse anatomical sites, with a distribution as follows: Extremities (68.7%), trunk (15.7%), head and neck (6.3%), intrathoracic (5.3%), intra-abdominal (1.8%), and miscellaneous (2.2%). The occurrence of SS in the head and neck region is extremely uncommon. Within this region, the hypopharynx is the most frequent site of occurrence. Other locations include the prevertebral and parapharyngeal areas, larynx, and nasopharynx. Rarely, SS has also been reported to arise from pleuropulmonary, cardiac, GI tract, and other body organs.[1,2]
SS is a misnomer, as they do not arise from synovium and are rarely intra-articular. This tumor does not exhibit synovial differentiation but instead shows epithelial characteristics. It stains for epithelial markers such as epithelial membrane antigen and cytokeratin, unlike synovium. Although the pathogenesis remains incompletely understood and no definitive cell of origin has been identified, SS is primarily attributed to a t(X:18) translocation. This translocation results in the fusion of the SYT gene (at 18q11) with the SSX1 or SSX2 genes (at Xp11), which is present in 95% of tumors.[3,4]
Histologically, SS is categorized into two main subtypes: monophasic and biphasic. The monophasic subtype, which is the most common, can either be the spindle cell type or the rare epithelial subtype. The biphasic subtype consists of both fibroblast-like, spindle-shaped cells, and epithelial cells. In addition, rare subtypes such as poorly differentiated (round cell), ossifying, and myxoid variants have also been identified. Tumor size correlates with histological characteristics: monophasic SSs are typically smaller than 5 cm, while biphasic SSs are usually larger than 5 cm.[3]
Head-and-neck SS commonly present as a painless, slow-growing asymptomatic mass until it grows large enough to compress surrounding structures. Symptoms of head and neck SS based on compression of near by structures, including mild throat discomfort, hoarseness, dysphagia and dyspnea.[4]
Diagnosing SS in head-and-neck region is clinically challenging. However, a high index of suspicion is warranted if a previously healthy adolescent or young adult presents with a hard mass in the extremity or elsewhere in the body.
DL and fiberoptic laryngoscopy are essential procedures used to assess the status of the mucosal lining in the larynx and pharynx.
SSs are submucosal in origin, so during laryngoscopic examination, the pharyngeal and laryngeal mucosa remains intact, and the vocal cords usually maintain normal mobility. The imaging features are highly variable and depend on the anatomic origin. Most lesions present as large aggressive heterogeneous masses containing hemorrhagic and cystic foci, often with calcification. However, up to one-third of lesions have commonly benign features and can appear homogeneously solid or cystic.
Computed tomography (CT) is useful for evaluation of cartilage erosion, airway compromise, and compression. CT typically shows a non-infiltrative, well-circumscribed mass, often with punctate, and peripheral calcifications. CECT shows mild heterogenous enhancement of the solid components.
MRI is the preferred modality for diagnosing and initially staging SS due to its detailed intrinsic signal characteristics and superior soft-tissue contrast. It is also used for local staging of the tumor.[2,5,6]
SSs exhibit a range of MRI appearances, from small, homogeneous nodules with minimal mass effect to large, heterogeneous masses encasing vessels and nerves.
SS can be purely cystic, homogeneous solid, and mixed solid/cystic. It can have hemorrhagic and calcific foci are often present. It appears iso to slightly hyperintense on T1-weighted images and predominantly hyperintense on T2-weighted images and on post contrast images, it shows diffuse (40%), heterogeneous (40%), or peripheral (20%) enhancements.[2,7]
Several characteristic signal patterns for large masses on T2-weighted imaging have been described. These include the “bowl of grapes” appearance, reflecting a multicystic mass with variable fluid intensities and fluid-fluid levels, and the “triple sign,” which indicates a mass with areas of hyperintensity, isointensity, and hypointensity relative to skeletal muscle. Hyperintensity is due to necrosis and cystic degeneration and hyperintensity is due to calcification and fibrous bands. MRI is optimal for assessing aggressive features such as adjacent bone involvement, neurovascular encasement, peritumoral edema, and muscular invasion.
Dynamic contrast-enhanced MRI, which provides insights into tumor microcirculation by reflecting angiogenesis, shows that early enhancement – a characteristic consistently observed in SS – should prompt consideration of SS in the differential diagnosis, even for nonspecific or small, well-defined, and benign-appearing masses.[1,4] MRI is also effective in post treatment response assessment, and they are often performed to detect early signs of recurrence. Histological examination remains necessary to differentiate between benign and malignant soft-tissue tumors.
Neoadjuvant chemotherapy and radiation therapy are commonly used for treating primary SSs. MRI can show a treatment response, typically seen as a reduction in tumor size. It might also increase in size due to necrosis development despite a positive treatment response. Furthermore, routine chest radiographs and CT scans are used to monitor potential pulmonary metastases.[8]
DIFFERENTIAL DIAGNOSIS
Undifferentiated pleomorphic sarcoma
Leomyosarcoma
Rhabdomyosarcoma,
Fibrosarcoma
CONCLUSION
SS of hypopharynx is a complex, aggressive soft-tissue malignancy, commonly affecting adolescents and young adults and frequently presenting in the extremities. MRI is crucial for diagnosis, staging, and monitoring treatment response, offering detailed insights into tumor characteristics and potential metastases. Early detection and comprehensive imaging evaluation are essential for guiding effective treatment and improving patient outcomes.
TEACHING POINTS
SSs often present as heterogeneous masses with distinct MRI characteristics such as the “bowl of grapes” and “triple sign.” Early enhancement on dynamic contrast-enhanced MRI can aid in distinguishing them from benign masses.
Despite their name, SSs rarely originate from synovial tissue and instead display epithelial characteristics, primarily due to a t(X:18) translocation. Diagnosis often relies on MRI for staging and monitoring, while histology differentiates between benign and malignant tumors.
MCQs
-
Which one of the following sentences is false regarding SS?
SS is the third most common soft tissue tumor.
Associated with cytogenetic aberration of the t(X: 18) translocation.
Most common location is extremities.
SSs arise from synovium.
Answer Key: d
-
What are the standard modalities for imaging of SS?
Ultrasound
PET-CT
CECT
MRI
All of the above
Answer Key: e
-
Which one of the following is correct?
SS contains dystrophic calcifications
Smaller tumors are associated with poor prognosis
Larger ones are associated with good prognosis
Synovial in older patients has good prognosis.
Answer Key: a
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Synovial sarcoma: Imaging features of common and uncommon primary sites, metastatic patterns, and treatment response. Am J Roentgenol. 2012;199:W208-15.
- [CrossRef] [PubMed] [Google Scholar]
- The imaging spectrum of synovial sarcomas: A pictorial review from a single-centre tertiary referral institution. Can Assoc Radiol J. 2021;72:470-82.
- [CrossRef] [PubMed] [Google Scholar]
- From the archives of the AFIP: Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26:1543-65.
- [CrossRef] [PubMed] [Google Scholar]
- Biphasic synovial sarcoma of the hypopharynx: Case report and literature review. Int J Surg Case Rep. 2022;91:106784.
- [CrossRef] [PubMed] [Google Scholar]
- Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2:39-41.
- [CrossRef] [PubMed] [Google Scholar]
- Synovial sarcoma in children: Imaging features and common benign mimics. Am J Roentgenol. 2010;195:1026-32.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging of soft tissue sarcoma: Features related to prognosis. Eur J Orthop Surg Traumatol. 2021;31:1567-75.
- [CrossRef] [PubMed] [Google Scholar]
- Synovial sarcoma: Characteristics, challenges, and evolving therapeutic strategies. ESMO Open. 2023;8:101618.
- [CrossRef] [PubMed] [Google Scholar]








