Translate this page into:
Exploring the enigmatic giant liver lesion
*Corresponding author: Venkatesh Kasi Arunachalam, Department of Radiology, Kovai Medical Center and Hospitals, Coimbatore, Tamil Nadu, India. radvenki79@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sugumar R, Kasi Arunachalam V, Gowtham SM, Rajasekaran S, Mehta SS. Exploring the enigmatic giant liver lesion. Case Rep Clin Radiol. doi: 10.25259/CRCR_51_2024
Abstract
Primary hepatic neuroendocrine tumor is a very rare lesion which poses a diagnostic challenge to the clinician as well as the radiologist due to various close mimics. Prompt identification, diagnosis, and treatment are imperative to save the life of the patient. In this case report, we wish to highlight the features of this rare entity in a 63-year-old male who presented with melena, weight loss, and decreased appetite.
Keywords
Neuroendocrine tumor
Functional neuroendocrine tumor
Carcinoid
Liver
INTRODUCTION
Primary hepatic neuroendocrine tumors (PHNETs) are exceptionally uncommon, unlike metastatic neuroendocrine tumors. With imaging alone, it is extremely challenging to differentiate this from other benign and malignant masses, notably hepatocellular carcinoma (HCC), hepatic adenoma, FNH, and cholangiocarcinoma.[1,2] Thus, distinguishing PHNETs radiologically from other liver carcinomas poses a significant challenge.
CASE REPORT
A 63-year-old male presented with complaints of melena, weight loss, decreased oral intake, and early satiety. He had been an alcoholic for 20 years and had a history of laparoscopic surgery for inguinal hernia repair. The initial work-up showed elevated calcium levels and low hemoglobin. No other complaints were reported. The patient was evaluated with abdominal ultrasonography (USG), contrast-enhanced computed tomography (CECT), and positron emission tomography – computed tomography (PET-CT).
Abdominal ultrasound [Figure 1] revealed a large, predominantly hypoechoic lesion in the right liver lobe with significant increased intralesional vascularity.
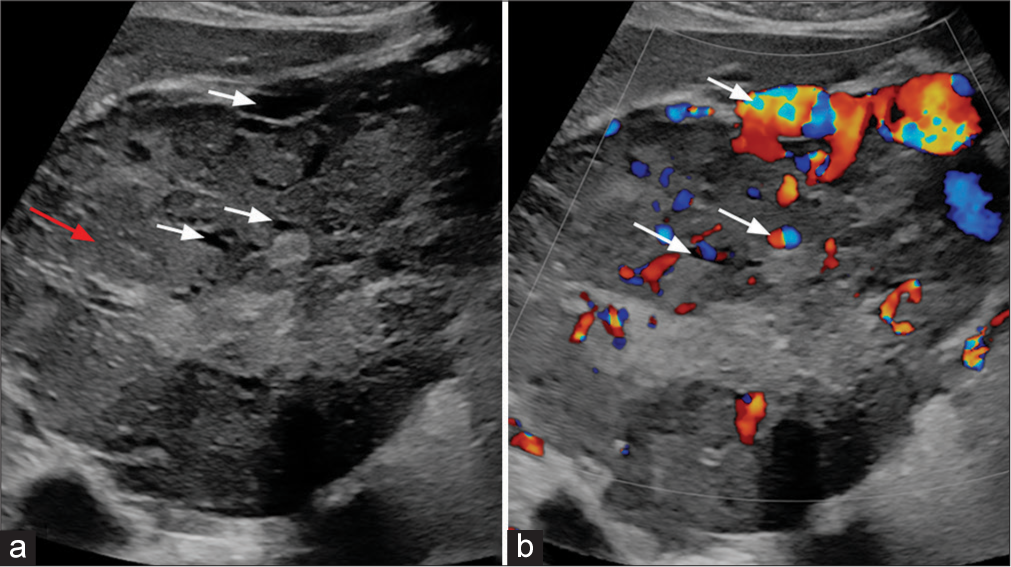
- (a) B-Mode ultrasound image of liver shows a large heterogeneous lesion (red arrow) with multiple anechoic areas (white arrows). (b) Color Doppler image of liver shows a intralesional vascularity (white arrows).
On CECT [Figure 2], predominant peripheral arterial enhancing lesion with central non-enhancing necrotic area was noted in the right lobe of liver. The lesion measures about 16 × 11.5 × 13.8 cm (TR × AP × CC) in maximum dimension. Focus of calcification also noted within the lesion. Dilated hepatic artery with multiple arterial shunting and multiple dilated and tortuous vessels along the periphery of the lesion was seen. Two other focal lesions with similar enhancement were seen along the periphery of the liver. All these lesions show washout on venous and delayed phase images. No evidence of surface nodularity or volume redistribution was seen to suggest cirrhosis. Based on these imaging features, possibility of malignant neoplasm (HCC/Hepatocholangio carcinoma/neuroendocrine tumor) was given and was evaluated further with PET CT and tru-cut biopsy. GALLIUM-68 DOTANOC PET CT [Figure 2] revealed avid tracer uptake within the abovementioned lesions. Under USG guidance, tru-cut biopsy was performed from the lesion and sent for further evaluation. Histopathology examination and immunohistochemical images [Figure 3] revealed features of neuroendocrine tumor. The patient is treated with octreotide. Follow-up PET CT done after 4 months showed interval decrease in the tracer uptake in the liver lesions.
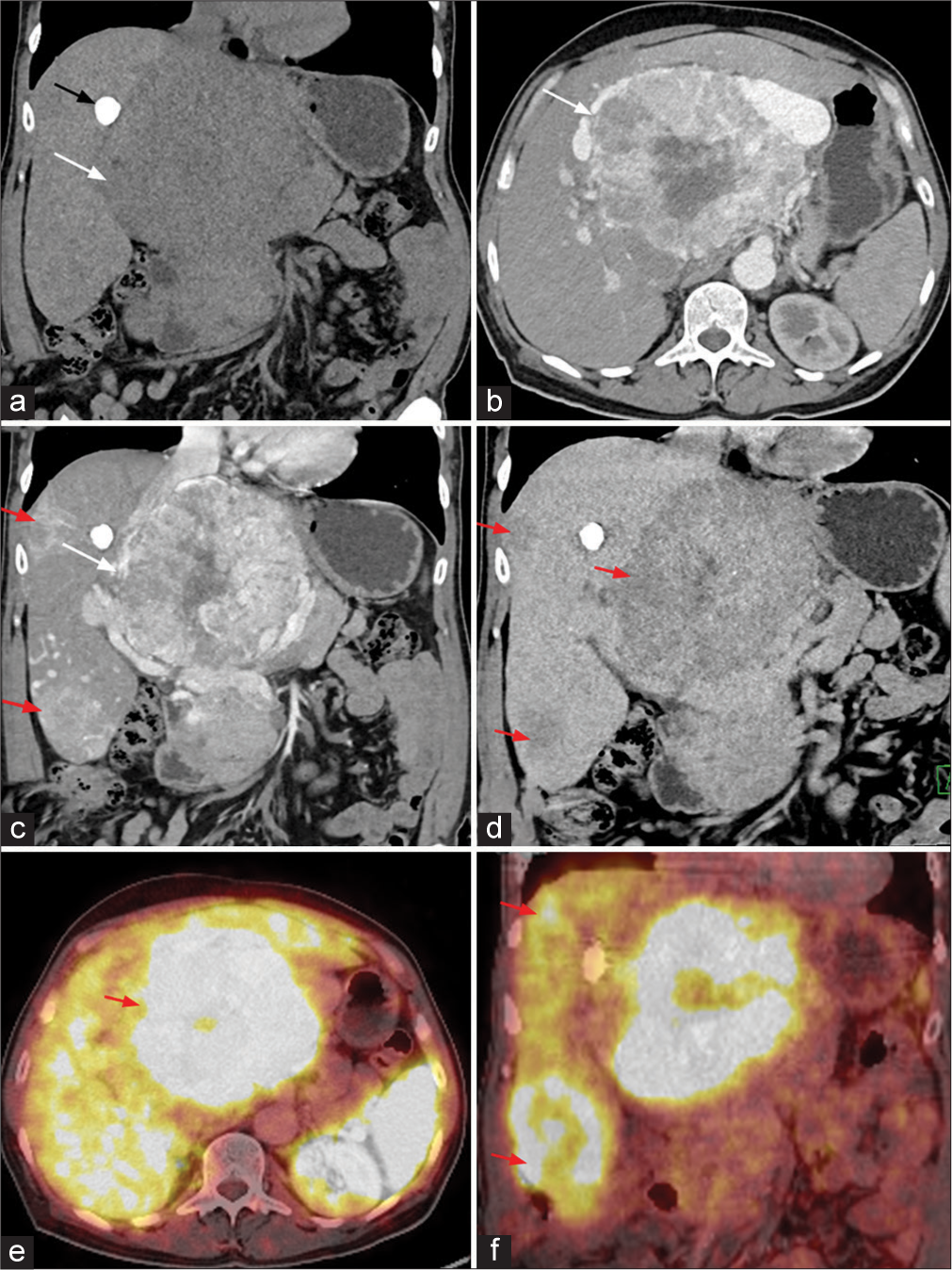
- (a-d) Contrast-enhanced computed tomography (CT) image of the abdomen (a) coronal plain CT image shows a large heterogeneous lesion in the region of porta hepatis (white arrow) with focus of calcification (black arrow) along the margin of the lesion. (b) Arterial phase image shows a heterogeneous hyperenhancement of the lesion (white arrow). (c) Coronal image in the arterial phase shows a largest lesion in the porta hepatis region (white arrow). Similar lesions were also demonstrated in the subcapsular location in the right lobe of liver (red arrows). (d) All the lesions show washout on delayed phase (red arrows). (e) Axial and (f) coronal Ga-68 DOTONOC PET CT image shows intense tracer uptake in the lesion in region of porta hepatis (red arrow in e) and lesions in subcapsular region of the right lobe of liver (red arrows in f).
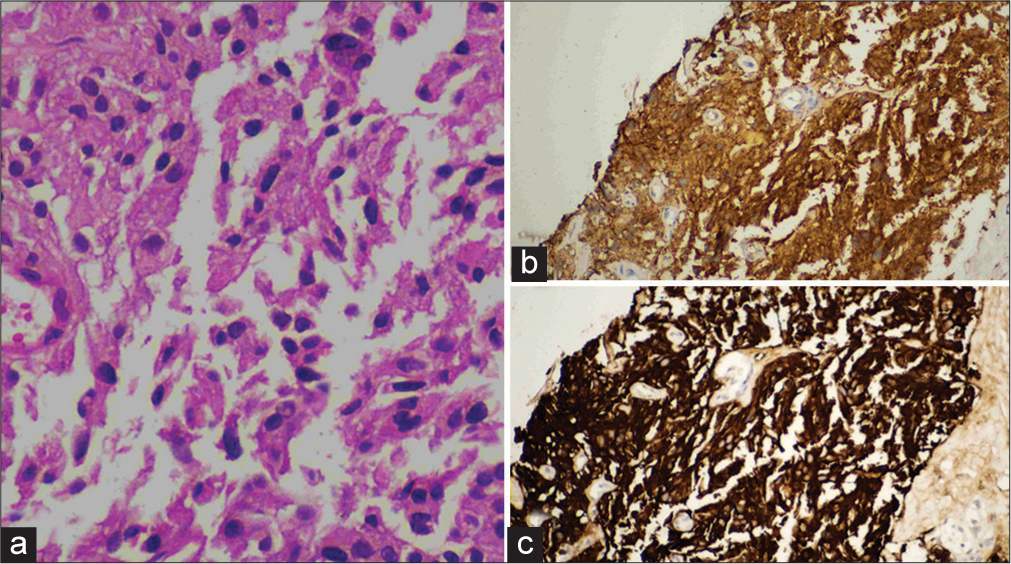
- (a) Histopathology image shows a nests and loose clusters of relatively monomorphous epithelial cells exhibiting ill-defined cell borders, moderate granular eosinophilic cytoplasm, and ovoid nuclei with coarse chromatin and inconspicuous nucleoli. These cell clusters are separated by fibrous septa. There is no necrosis. (b and c) Immunohistochemical image shows positive for neuron specific enolase and synaptophysin markers.
DISCUSSION
The neuroendocrine system includes neuroendocrine cells derived from the embryonic neural crest, neuroectoderm, and endoderm, which includes adrenal medulla, pancreatic islets, paraganglia, parathyroid, pituitary, and thyroid C cells. They are widely dispersed throughout the body including the gastrointestinal tract, biliary tract, liver, lung, urethra, and skin. As a result, neuroendocrine tumors have the potential to involve various organs and sites where these cells are present, commonly manifesting within the gastroenteropancreatic axis. The most common sites involved are the bowel, pancreas, and lung.[3,4]
They usually produce and release bioactive substances (i.e., Serotonin, histamine, and tachykinins). When these tumors cause a clinical syndrome due to the release of bioactive substances, they are termed as functioning tumors. Such tumors typically manifest early with noticeable symptoms, presenting a challenge for radiologists in terms of localization because they are often small. Non-functioning tumors are characterized by either the absence of hormone secretion or the presence of hormone secretion that does not lead to any identifiable clinical syndrome. These are often discovered incidentally during imaging studies, due to mass effects, or on presentation with metastatic disease. Neuroendocrine tumors (NETs) exhibit a broad spectrum of biological behaviors ranging from slow growing to aggressive behavior.
In 2000, the World Health Organization (WHO) issued a review of the nomenclature and classification of neuroendocrine tumors. It has been recommended to substitute the term “carcinoid” with “endocrine” or “neuroendocrine carcinoma” to lessen the ongoing confusion prevalent in literature. Based on pathological criteria of morphology, proliferative index, presence of vascular invasion, and WHO classified neuroendocrine tumors into three grades, it includes.[3]
Well-differentiated neuroendocrine carcinoma (corresponding to classical carcinoid)
Moderately differentiated neuroendocrine carcinoma (corresponding to atypical carcinoid)
Poorly differentiated neuroendocrine carcinoma (corresponding to small cell carcinoma).
NETs are considered as the rarest subtypes, accounting for <1% of primary liver tumors that undergo surgical resection. Typically, liver involvement occurs mostly due to the metastasis of NETs. PHNETs, a unique and infrequent subtype of NETs, originate within the intrahepatic bile duct epithelium, demonstrating an exceptionally low incidence rate of 0.3%.[5]
PHNETs are frequently portrayed as having slow growth rates and lacking functional hormone secretion in the majority of documented cases. These tumors have no gender predominance and the majority tend to primarily affect individuals within the age range of 40–50 years old. The majority of PHNETs have been documented in the right lobe of the liver than the left lobe.[5,6]
PHNETs are discovered incidentally in routine screenings. Most of the PHNET are non-functional and grow asymptomatically. Carcinoid syndrome was observed <10% of the cases. They usually present with abdominal pain, abdominal distension, bloating, weight loss, jaundice, and tiredness. Hepatomegaly is observed in advanced cases. Serum tumor markers such as alpha-fetoprotein and carcinoembryonic antigen are not clinically useful for diagnosing PHNETs.[5,6]
A key diagnostic criterion for PHNETs is the lack of lesions in other typical sites for this tumor type, such as the small intestine, pancreas, and lungs.[5] Thus, when identifying an NET in the liver, it is crucial to search for a primary site outside of the liver. The rarity of this condition results in a lack of prospective data, posing challenges in determining the appropriate diagnostic and treatment strategies.
Typically, these lesions are single and heterogeneous, demonstrating a hypervascular pattern of enhancement on both the arterial and portal phases that is more prominent at the periphery and delayed enhancement at the center. They may exhibit cystic regions (necrosis) and fluid-fluid levels. They often display a primary lesion encircled by multiple satellite nodules.[7]
One characteristic of PHNETs is arterial phase hyperenhancement, attributed to the abundant blood supply from the hepatic artery. The primary lesion commonly presents discontinuous and irregular enhancements of the capsule. However, differentiating PHNETs from other conditions such as primary HCC, cholangiocarcinoma, hepatic adenoma, and FNH pose a challenge, as they all receive their blood supply from the hepatic artery.[8,9]
DIFFERENTIAL DIAGNOSIS
The closest differential diagnosis and their salient imaging features are mentioned in Table 1.
| Hepatic adenoma | • Most common in young females with history of oral contraceptive pill consumption |
| • Arterial phase hyperenhancement with isodense to liver parenchyma in portal venous phase | |
| Focal nodular hyperplasia | • Arterial phase hyperenhancement with centrifugal filling pattern. |
| • Isodense to liver parenchyma in portal venous phase | |
| • Prominent feeding vessel | |
| • Non-enhancing central scar | |
| Primary HCC | • Most common in cirrhotic liver |
| • Arterial phase hyperenhancement with washout in portal venous phase | |
| • Delayed capsular enhancement | |
| Mass forming cholangiocarcinoma | • Patient with obstructive jaundice. |
| • Peripheral enhancement in arterial phase with gradual progressive centripetal enhancement in portal venous and delayed phases | |
| • Adjacent capsular retraction and distal IHBR | |
| Metastasis | • Metastases are hypodense on unenhanced computed tomography (CT) and hypoenhancing on contrast study |
| • Enhancement is typically peripheral in arterial and portal venous phase with washout in delayed phase |
PHNET: Primary hepatic neuro endocrine tumor, HCC: Hepato cellular carcinoma, IHBR: Intra hepatic biliary radicles dilatation
CONCLUSION
PHNETs are uncommon and asymptomatic tumors, posing challenges in radiological differentiation from other liver carcinomas. In cases of incidentally detected hypervascular liver mass, where patients lack clinical symptoms, exhibit normal tumor marker levels, show suspected malignant tumor features without typical imaging manifestations, and absence of similar lesions elsewhere in the body, PHNETs should be considered in the differential diagnosis.
TEACHING POINTS
PHNET is considered as the rarest subtypes, accounting for <1% of primary liver tumors that undergo surgical resection
A focal lesion with hyperenhancement in arterial phase, multiple satellite lesions, and predominant peripheral enhancement, absent hyperenhancing lesions elsewhere (Pancreas, intestine, and lungs) favor PHNET.
MCQs
-
Which one of the following sentences is true regarding PHNET?
Presence of lesions in other organs.
Hyperenhancement of lesion in post-contrast delayed phase images
Absence of lesions in other organs
Most commonly present with carcinoid syndrome.
Answer Key: c
-
What is the incidence of carcinoid syndrome in primary neuroendocrine tumor of liver?
<10%
30–40%
100%
Never presents as carcinoid syndrome.
Answer Key: a
-
Which one of the following is correct?
Functional neuroendocrine tumor present in late stages
Non-functional NET present in early stages
Non-functional NET secretes bioactive amines
Functional NET secretes bioactive amines
Answer Key: d
-
What is the cell of origin for PHNET?
Biliary epithelial cells
Hepatocyte
Kuffer cells.
Both a and c
Answer Key: a
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Primary hepatic neuroendocrine tumor: A case report and literature review. World J Clin Cases. 2016;4:243.
- [CrossRef] [PubMed] [Google Scholar]
- Primary neuroendocrine tumor of liver (rare tumor of liver) Iran J Cancer Prev. 2015;8:e3144.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroendocrine neoplasms of the gastrointestinal tract: Classification, pathologic basis, and imaging features. RadioGraphics. 2007;27:1667-79.
- [CrossRef] [PubMed] [Google Scholar]
- Primary hepatic neuroendocrine tumors: Multi-modal imaging features with pathological correlations. Cancer Imaging. 2017;17:20.
- [CrossRef] [PubMed] [Google Scholar]
- Primary hepatic neuroendocrine tumor: A rare entity. Radiol Case Rep. 2020;15:2362-6.
- [CrossRef] [PubMed] [Google Scholar]
- The outcome of primary hepatic neuroendocrine tumors: A single-center experience. J Clin Exp Hepatol. 2019;9:710-5.
- [CrossRef] [PubMed] [Google Scholar]
- Primary hepatic neuroendocrine tumors: Comparing CT and MRI features with pathology. Cancer Imaging. 2015;15:13.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic neuroendocrine neoplasm: Imaging patterns. Radiol Bras. 2020;53:195-200.
- [CrossRef] [PubMed] [Google Scholar]
- Focal nodular hyperplasia and focal nodular hyperplasia-like lesions. RadioGraphics. 2022;42:1043-61.
- [CrossRef] [PubMed] [Google Scholar]







