Translate this page into:
A rare case of persistent mullerian duct syndrome with adenocarcinoma of ascending colon
*Corresponding author: Vaibhavkumar Maganbhai Vekaria, Department of Radiology, Dr. M. K. Shah Medical College and Research Center, Ahmedabad, Gujarat, India. vaibhavvekaria16@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Patel NG, Patel S, Vekaria VM, Sharma H, Banugariya CP. A rare case of persistent mullerian duct syndrome with adenocarcinoma of ascending colon. Case Rep Clin Radiol. doi: 10.25259/CRCR_190_2023
Abstract
Persistent mullerian duct syndrome (PMDS) is a rare congenital disorder. This condition results from a failure of Mullerian duct regression during fetal development, leading to the presence of uterus, fallopian tubes, and upper vagina in otherwise phenotypically male individuals. Radiological evaluation plays a crucial role in the diagnosis and management of PMDS. Various imaging modalities including ultrasound, magnetic resonance imaging, and computed tomography are employed to visualize and confirm the presence of Mullerian structures in affected individuals.
Keywords
Persistent mullerian duct syndrome
Computed tomography
Adenocarcinoma
INTRODUCTION
Radiological imaging techniques have a significant role in guiding the management of Persistent Mullerian Duct Syndrome (PMDS). Ultrasound is often the initial imaging modality of choice due to its accessibility, cost-effectiveness, and lack of ionizing radiation. It can provide real-time imaging and identify the presence of a uterus, fallopian tubes, and upper vagina. In addition, ultrasound can help evaluate associated abnormalities such as testicular maldescent, which may be present in conjunction with PMDS. Magnetic resonance imaging (MRI) is a valuable tool for further characterization of the Mullerian remnants.
CASE REPORT
A 25-year-old phenotypic male patient presented with a complaint of abdominal pain associated with anorexia, intermittent fever, and blood in stool for 6 months. The patient also had operated for hypospadias in the past 5 times. The patient had single descended testes on clinical examination of the scrotum. The patient’s vitals were stable. After written informed consent and proper sedation, the patient was taken for colonoscopy and a biopsy was taken from the mass lesion visualized in the ascending colon and sent to histopathology laboratory. The histopathological report suggested moderately differentiated adenocarcinoma.
Imaging findings
Ultrasonography (USG) pelvis revealed a uterus-like structure – [Figure 1a], which can also be seen on plain computed tomography (CT) in sagittal section – [Figure 1b] and pelvic MRI sagittal T2-weighted section – [Figure 1c]. Contrast enhanced computed tomography (CECT) abdomen revealed heterogeneously enhancing colonic mass in the ascending colon in Figure 1d.

- A 25-year-old phenotypic male patient presented with complaint of abdominal pain. (a) Pelvic USG showing uterus like structure (orange arrow), (b) sagittal section of pelvic computed tomography scan showing uterus like structure (orange arrow). (c) Sagittal section of pelvic magnetic resonance imaging T2-weighted image showing uterus like structure (orange arrow). (d) Axial section of contrast-enhanced computed tomography image showing colonic mass (orange arrow).
After the written informed consent, the patient was taken for laparotomy with right-sided hemicolectomy with subsequent removal of hypo-plastic uterus and tubo-ovarian structure. The excision biopsy was sent to the histopathology laboratory.
The histopathological report
Rudimentary uterus with fallopian tube – [Figure 2a]
Right hemicolectomy specimen [Figure 2b]: Poorly differentiated adenocarcinoma of ascending colon.
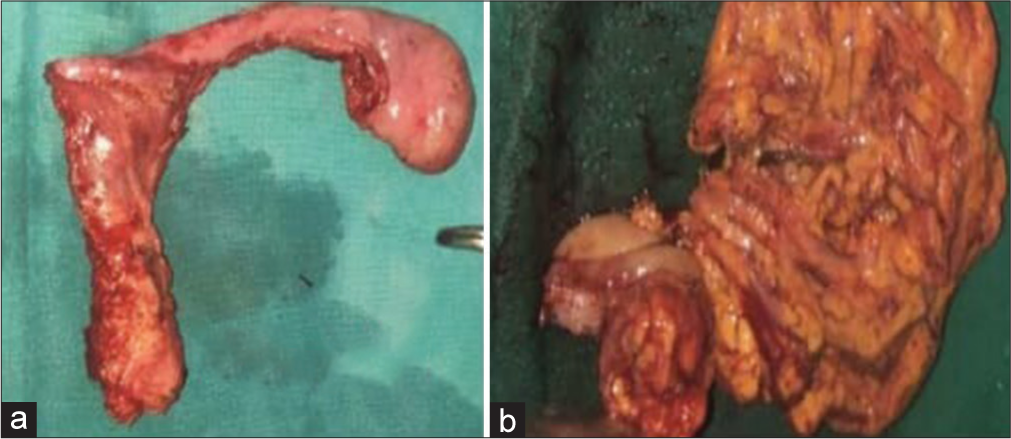
- A 25-year-old phenotypic male patient presented with complaint of abdominal pain. (a) Gross specimen image of resected rudimentary uterus, (b) Gross image of resected hemicolectomy specimen.
The karyotyping of the patient suggested 46 + X chromosomes with the presence of large marker chromosome and no evident Y chromosome.
Genotype of the patient was female type with a single X chromosome.
DISCUSSION
This case represents a rare form of pseudohermaphroditism in males, which is caused by a deficiency in the intrauterine release of anti-Mullerian hormone (AMH),[1] and it has an autosomal recessive mode of transmission, with few rare cases that had the possibility of familial X-linked inheritance.[2] There are female genital organs such as uterus, fallopian tubes, and upper one-third of the vagina present in a phenotypical male derived from the Mullerian duct.[3]
Mullerian and Wolffian ducts are both present in the fetus in the 7th week of gestation. The Wolffian duct will develop male reproductive organs; subsequently, the Mullerian duct will be regressed and will not develop from the AMH released from the Sertoli cells of the male fetus.[1] If the AMH is not released, there will be development of both male and female reproductive organs that are derived from the Wolffian and Mullerian duct, respectively.[3,4] PMDS has male and female types. The male type is most common.[5]
Types of PMDS and associated genetic mutations
There are two types of PMDS. Type I is associated with AMH gene mutation on chromosome 19 which is responsible for the synthesis of anti-Mullerian hormone. Type II is associated with mutation of AMHR gene mutation on chromosome 12 which is AMH receptor gene. Type I is more common than type II.
Clinical significance
The patients of PMDS are more likely to develop testicular malignancy in which seminomas are most common.[5] Furthermore, there are reports of malignant transformation of Mullerian remnants. There are no significant other reports of increased risk of colonic carcinoma in the patients of PMDS.
The testicular anomalies associated with PMDS are of three types
Female type: Both testes are intra-abdominal in location along the Mullerian duct structures
Male type: Hernia uteri inguinale: One testis is in inguinal hernia sac or hemi-scrotum and the other one is along the Mullerian duct structures in the abdomen
Transverse testicular ectopia: Both testes are in a single hemi scrotum alongside Mullerian duct structures.[6]
Management and follow-up
It includes surgical removal of the Mullerian duct structures as they could undergo malignant changes as well as hypertrophic and/or hemorrhagic changes with recurrent urinary tract infection, stones, and voiding disturbances. Since there is an increased risk of developing testicular malignancy, these patients have to be followed up for screening for the same if the clinician decides to preserve fertility. Surgeon can decide to perform an orchidectomy if testis undergoes malignant changes in follow-up screening scans. Ultrasound of the pelvis is usually an initial modality implemented and can also be used as a screening modality for regular follow-up. MRI of the pelvis is a modality of choice to confirm the location of Mullerian duct structures.[7,8]
DIFFERENTIAL DIAGNOSIS
Most common differential diagnosis of PMDS are Mixed gonadal dysgenesis, Herniated bowel loops and Scrotal cystocele. Pelvic ultrasound and MRI can be used to rule out the differential diagnosis. The imagine differentiating features between mixed gonadal dysgenesis and PMDS are mentioned in Table 1. Mixed gonadal dysgenesis would show unilateral testes with contralateral streak of testes. Herniated bowel loops can be confirmed on ultrasound by visualization of hernia sac and its contents. Scrotal cystocele can be confirmed on ultrasound of scrotum in which a part of urinary bladder herniates into scrotum.
| Differential diagnosis | Differentiating features |
|---|---|
| Mixed gonadal dysgenesis | Unilateral testes, contralateral streak of testes, appears T2 hyperintense streak on MRI |
| Persistent Mullerian duct syndrome | Rudimentary uterus, fallopian tubes, and upper third of vagina can be visualized on ultrasound and MRI of the pelvis. |
PMDS: Persistent mullerian duct syndrome
CONCLUSION
Persistent Müllerian Duct Syndrome exemplifies the importance of radiology in unraveling complex congenital anomalies, emphasizing the need for interdisciplinary approach to provide comprehensive care for patients. Further research and advancements in imaging technologies will enhance our understanding of PMDS and contribute to improved diagnostic accuracy and outcomes in the future.
TEACHING POINTS
This being a rare condition with <100 cases reported, the diagnosis can often be difficult
Primary infertility should raise suspicion of this condition, which then requires further investigations and definite treatment to limit the complications like hemorrhagic changes within the uterus and urinary tract infections.
MCQs
-
Which imaging technique is preferred for assessing the internal anatomy of Mullerian remnants in PMDS?
Plain radiography
Ultrasonography
Magnetic resonance imaging (MRI)
Fluoroscopy
Answer Key: c
-
What is the role of contrast-enhanced imaging in PMDS evaluation?
Identifying uterine anomalies
Assessing vascularity in Mullerian remnants
Differentiating between vas deferens and Mullerian remnants
Measuring prostate volume
Answer Key: b
-
Which radiological parameter is crucial in planning surgical interventions for PMDS?
Size of the seminal vesicles
Thickness of the prostate capsule
Location of Mullerian remnants
Uterine wall thickness
Answer Key: c
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Anti-mullerian hormone in early human development. Early Hum Dev. 1993;33:91-9.
- [CrossRef] [PubMed] [Google Scholar]
- Persistence of mullerian derivatives in males. Am J Med Genet. 1999;89:218-23.
- [CrossRef] [Google Scholar]
- Embryology and endocrinology of genital development. Ballieres Clin Endocrinol Metab. 1998;12:17-33.
- [CrossRef] [PubMed] [Google Scholar]
- The management of the persistent müllerian duct syndrome. Arab J Urol. 2014;12:239-44.
- [CrossRef] [PubMed] [Google Scholar]
- The persistent müllerian duct syndrome: An update based upon a personal experience of 157 cases. Sex Dev. 2017;11:109-25.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent müllerian duct syndrome: How to deal with the müllerian duct remnants, a review. Indian J Surg. 2010;72:16-9.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent mullerian duct syndrome. Radiographics. 2003;23:309-13.
- [CrossRef] [PubMed] [Google Scholar]
- Radiological findings in persistent müllerian duct syndrome: Case report and review of literature. J Radiol Case Rep. 2017;11:7-14.
- [CrossRef] [PubMed] [Google Scholar]







