Translate this page into:
Unusual pineal gland lesion presenting with obstructive hydrocephalus: A multimodal imaging perspective
*Corresponding author: Azadeh Eslambolchi, Department of Nuclear Medicine, Washington University in St Louis, Saint Louis, United States. azadeh@wustl.edu
-
Received: ,
Accepted: ,
How to cite this article: Eslambolchi A, Marjai S. Unusual pineal gland lesion presenting with obstructive hydrocephalus: A multimodal imaging perspective. Case Rep Clin Radiol. doi: 10.25259/CRCR_27_2025
Abstract
Metastatic melanoma to the pineal gland is exceedingly rare, with only a few cases documented in the literature. We report a 41-year-old male who presented with progressive headaches, nausea, vomiting, and altered mental status, ultimately found to have obstructive hydrocephalus secondary to a hypermetabolic pineal mass. Imaging revealed a well-circumscribed lesion on positron emission tomography/computed tomography (standardized uptake value [SUV] of 17.6) and magnetic resonance imaging findings consistent with cerebrospinal fluid obstruction. Further evaluation identified additional metastatic sites, including a left upper quadrant skin lesion. Histopathologic examination confirmed metastatic melanoma with a primary cutaneous origin. Despite undergoing whole-brain radiation therapy and systemic immunotherapy, the patient’s neurological condition deteriorated due to progressive central nervous system disease. This case underscores the critical role of advanced imaging techniques in identifying atypical metastatic sites and highlights the challenges in managing rare intracranial melanoma metastases.
Keywords
Brain metastasis
Central nervous system
Melanoma
Metastasis
Obstructive hydrocephalus
Pineal gland
INTRODUCTION
Melanoma is an aggressive malignancy originating from melanocytes, with a high potential for metastasis, particularly to the central nervous system (CNS). Brain metastases occur in approximately 20% of metastatic melanoma cases, often indicating a poor prognosis. However, metastasis to the pineal gland is exceptionally rare, with only a few documented cases.[1]
The pineal gland, a small endocrine structure within the epithalamus, plays a crucial role in regulating circadian rhythms through the production of melatonin. Despite its rich vascular supply, including a lack of a blood-brain barrier, metastatic involvement remains uncommon. The gland’s deep-seated location and relatively small size may contribute to the rarity of clinically detected metastases. Nevertheless, when affected, pineal gland lesions can lead to obstructive hydrocephalus and associated neurological symptoms, complicating diagnosis and management.[2]
Here, we present a rare case of metastatic melanoma to the pineal gland, highlighting its diagnostic challenges and treatment considerations.
CASE REPORT
A 41-year-old male presented with progressively worsening headaches, nausea, vomiting, and altered mental status. He had no significant past history of any malignancy. Neurological examination revealed disorientation and bilateral papilledema, suggestive of raised intracranial pressure. Cognitive impairment was present, though no focal neurological deficits were identified.
Initial contrast-enhanced computed tomography (CT) of the brain [Figure 1] demonstrated a 22 × 21 × 18 mm intensely enhancing mass in the pineal region with a few peripheral coarse calcifications. The lesion caused partial obstruction of the cerebral aqueduct, resulting in mild dilatation of bilateral lateral and third ventricles with mild transependymal ooze. Contrast enhanced magnetic resonance imaging (MRI) was also performed for further localization and characterization of the lesion revealing a well-circumscribed, heterogeneously enhancing, T1 hyperintense, and T2 iso- to hypointense mass exerting a severe mass effect on the tectum and cerebral aqueduct, leading to obstructive hydrocephalus and resultant dilatation of bilateral lateral and third ventricles [Figure 2ac]. Given these imaging characteristics, possible differential diagnoses included primary pineal region tumors (e.g., germinoma, pineocytoma, and pineoblastoma), metastatic lesions, and vascular malformations. In addition, a focus of T1 hyperintensity was observed in the posterior right parietal lobe, the significance of which remained uncertain at this stage [Figure 2d and e].
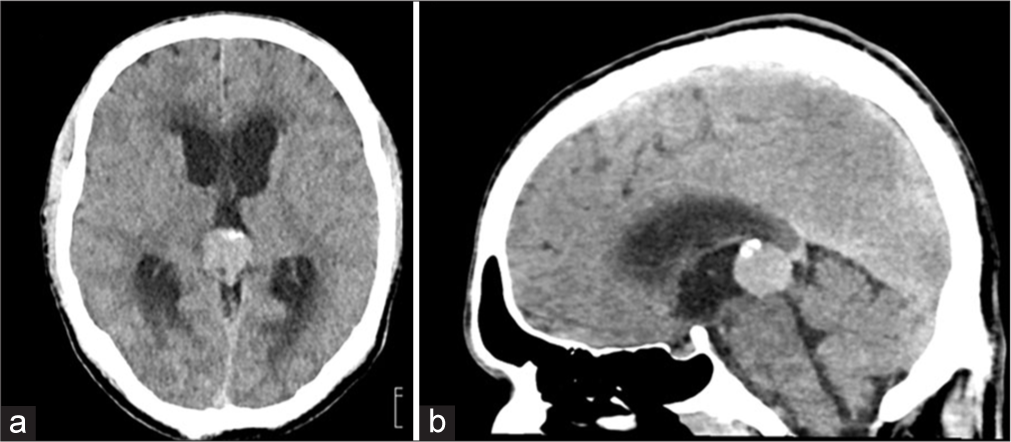
- Contrast-enhanced computed tomography of the brain in a 41-year-old man with metastatic melanoma to the pineal gland. (a) Axial and (b) sagittal views demonstrate a 22 × 21 × 18 mm enhancing mass in the pineal region, causing partial obstruction of the cerebral aqueduct. A few peripheral coarse calcifications are present. Probable mild transependymal cerebrospinal fluid flow from the ventricles is noted.
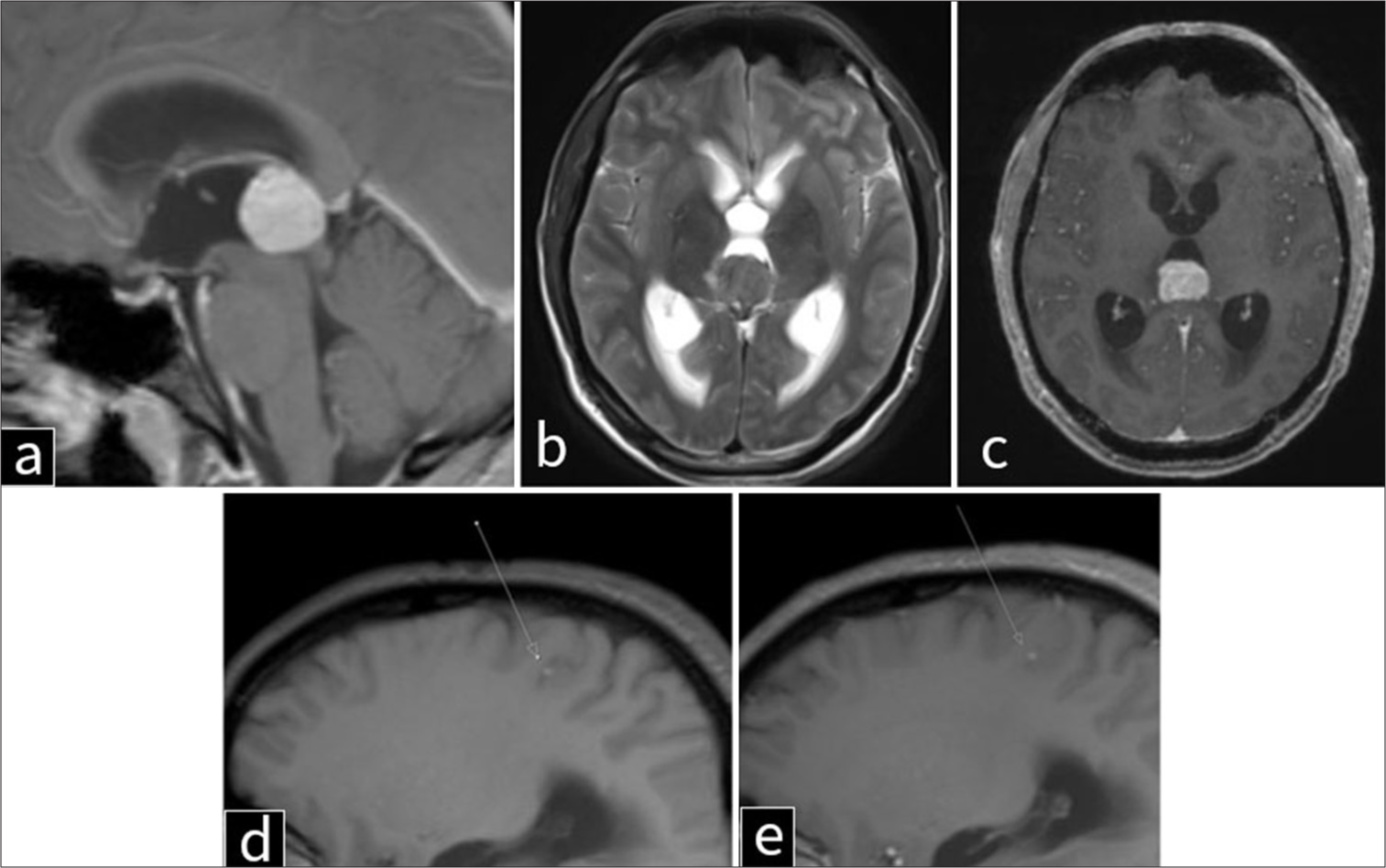
- Magnetic resonance imaging of the brain in a 41-year-old man with metastatic melanoma to the pineal gland. (a) T1-weighted sagittal, (b) T2-weighted axial, (c) post-contrast T1-weighted axial, (d) T1-weighted parasagittal, and (e) post-contrast T1-weighted parasagittal views of the brain demonstrate a well-circumscribed, T1-hyperintense pineal lesion resulting in severe mass effect on the tectum and cerebral aqueduct, with associated obstructive hydrocephalus of the lateral and third ventricles and transependymal cerebrospinal fluid flow. The mass appears to originate from the anterior aspect of the pineal region and measures 2.1 × 2.1 cm. Another focus of leptomeningeal T1 hyperintensity is noted in the posterior right parietal lobe (arrows in d and e).
Given the presence of an intracranial mass with features concerning for malignancy on MRI, positron emission tomography (PET) imaging was performed to assess metabolic activity, further characterize the primary source of disease, and evaluate for systemic metastatic involvement. PET imaging [Figure 3a-c] demonstrated significant hypermetabolic activity within the pineal mass, measuring slightly >2 cm, with a maximum SUV of 17.6. Additional fluorodeoxyglucose (FDG)-avid lesions included a 1.0 cm left retropectoral lymph node (SUV 7.3) [Figure 3d] and a 3 cm liver lesion in segment 7/8 (SUV 9.6) [Figure 3e], further supporting widespread metastatic disease. At the time of hospital admission for neurological symptoms, a skin lesion on the left upper abdomen was also noted. PET imaging revealed the lesion to be hypermetabolic, with a maximum SUV of 8.4 [Figure 3f].
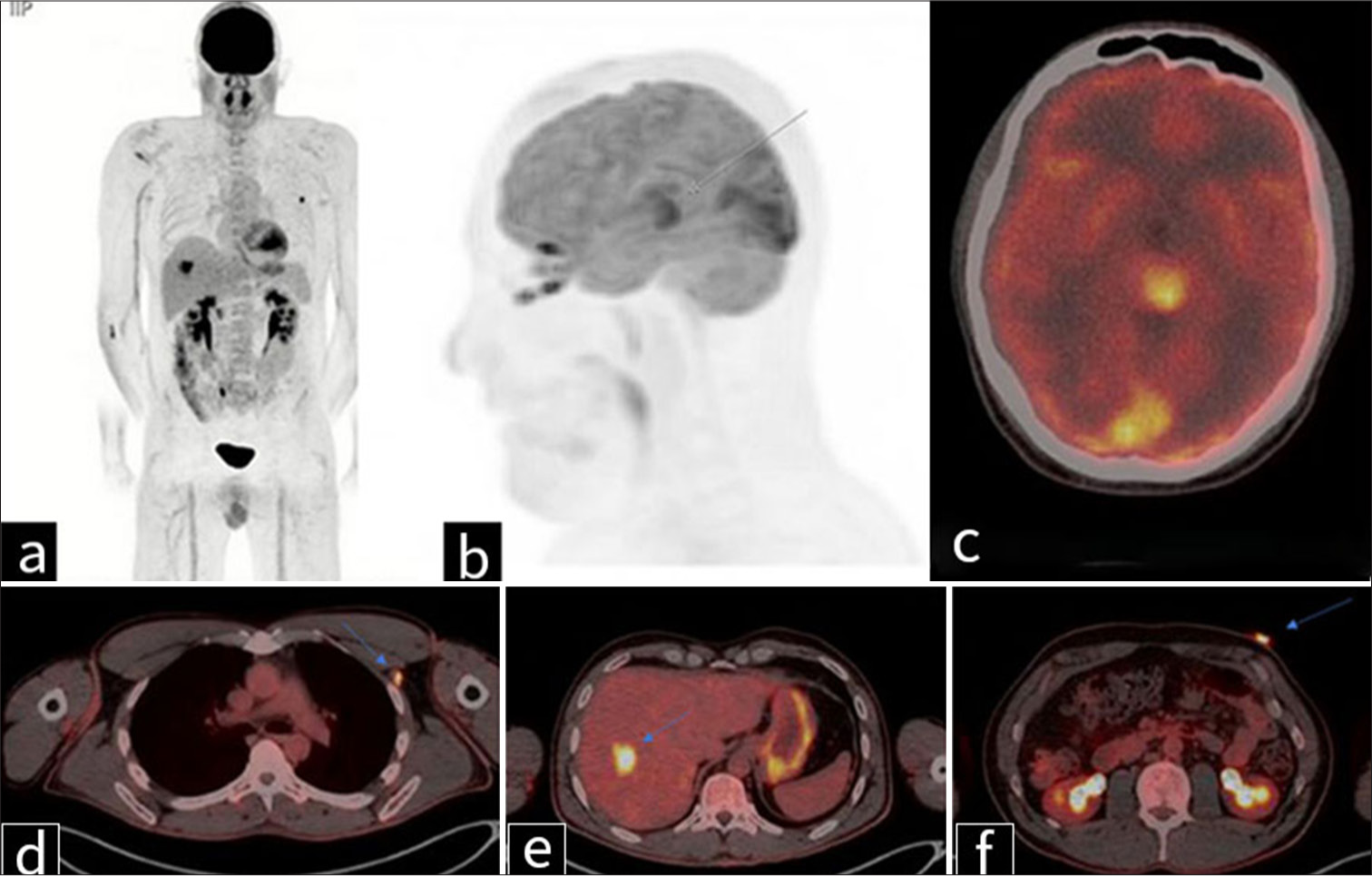
- Positron emission tomography imaging in a 41-year-old man with metastatic melanoma. (a) Coronal and (b) sagittal maximum intensity projection images, along with (c-f) transaxial fused images, demonstrate (c) a hypermetabolic pineal mass measuring slightly >2 cm, with a maximum standardized uptake value (SUV) of 17.6 (gray arrow in b). (d) A 1.0 cm markedly fluorodeoxyglucose-avid left retropectoral lymph node is noted, with a maximum SUV of 7.3 (blue arrow). (e) A liver lesion in segment 7/8 measures approximately 3 cm and has a maximum SUV of 9.6 (blue arrow). (f) A skin lesion in the left upper abdomen measures approximately 17 mm, with a maximum SUV of 8.4 – primary source of malignant melanoma (blue arrow).
A biopsy of both the left upper quadrant skin lesion was performed, confirming malignant melanoma as the primary source of metastasis. A stereotactic biopsy of the pineal gland lesion confirmed metastatic melanoma. Concurrently, a 2 cm left upper quadrant skin lesion was identified and biopsied, further supporting the diagnosis. A ventriculoperitoneal shunt was placed to relieve hydrocephalus. The patient subsequently underwent whole-brain radiation therapy and was started on systemic immunotherapy with checkpoint inhibitors. However, due to progressive CNS disease, palliative care was initiated, and the patient succumbed to his illness.
DISCUSSION
Given the rarity of pineal gland metastases, understanding the pathological and radiologic hallmarks of melanoma is crucial in differentiating it from primary pineal tumors. Melanoma arises from melanocytes, neural crest-derived pigment-producing cells primarily located in the skin but also present in mucosal surfaces, the uveal tract of the eye, and the leptomeninges. Histopathologically, melanoma is characterized by atypical melanocytes with large, pleomorphic nuclei, prominent nucleoli, and abundant cytoplasmic melanin pigmentation. Metastatic melanoma often demonstrates high mitotic activity, vascular invasion, and variable degrees of necrosis. The presence of melanin often leads to T1 hyperintensity on MRI, a hallmark feature aiding radiologic diagnosis.
Radiologically, melanoma metastases are unique in their imaging characteristics compared to other brain metastases. On contrast-enhanced MRI, they frequently appear as well-circumscribed, intensely enhancing lesions with intrinsic T1 hyperintensity due to melanin and hemorrhagic components. On T2-weighted imaging, signal intensity can be variable, ranging from iso- to hypointense, depending on the presence of melanin, hemorrhage, and necrosis. Susceptibility-weighted imaging (SWI) can further aid in detection by highlighting hemorrhagic foci. FDG-PET/CT plays a critical role in staging and assessing metabolic activity, with melanoma lesions demonstrating intense FDG uptake due to their high glycolytic metabolism. Given the propensity of melanoma for widespread hematogenous dissemination, imaging should include whole-body PET/CT to evaluate for extracranial metastatic disease.
Melanoma frequently metastasizes to the CNS due to its hematogenous dissemination and immune evasion mechanisms. While brain metastases are relatively common, involvement of the pineal gland is exceptionally rare, with fewer than 20 cases reported.[1] The pineal gland’s unique vascular supply and deep venous junction may facilitate metastatic spread. However, its small size and low metabolic activity likely contribute to the rarity of clinically detected metastases.[3] Symptoms of pineal metastases, such as headaches and altered mental status, are non-specific and overlap with other intracranial pathologies. Advanced imaging modalities, including FDG-PET/CT and contrast-enhanced MRI, are critical for distinguishing metastatic lesions from primary pineal tumors.[4] Management of pineal gland metastases is challenging due to their delicate location and the advanced stage at presentation. Treatment typically involves a combination of surgery, radiation therapy, and systemic immunotherapy. Despite aggressive management, prognosis remains poor, highlighting the need for novel therapeutic strategies.
DIFFERENTIAL DIAGNOSIS
-
Primary Pineal Region Tumors o Germ Cell Tumors (may have hemorrhagic components like melanoma)
o Germinoma (most common in young males)
o Choriocarcinoma (often highly hemorrhagic)
o Embryonal carcinoma
o Mixed germ cell tumor
o Pineal Parenchymal Tumors
o Pineocytoma
o Pineoblastoma (high-grade, aggressive)
o Pineal parenchymal tumor of intermediate differentiation
o Glial Tumors
o Astrocytoma (including glioblastoma)
o Ependymoma.
-
Metastatic Lesions
Lung cancer (most common source of brain metastases)
Breast cancer
Renal cell carcinoma
Gastrointestinal cancers (colorectal, gastric, pancreatic, etc.)
Other melanotic metastases (e.g., clear cell sarcoma and PEComa).
-
Vascular Lesions
Cavernous malformation (T1-hyperintense due to hemorrhage)
Arteriovenous malformation.
Primary CNS lymphoma (typically hyperdense on CT, enhances homogeneously).
-
Other Considerations
Pineal cyst (usually benign but can cause mass effect)
Neurosarcoidosis (can mimic metastatic lesions).[4]
CONCLUSION
This case highlights the rarity of metastatic melanoma to the pineal gland and its associated diagnostic and therapeutic challenges. Early recognition of atypical metastatic sites, advanced imaging techniques, and a multidisciplinary treatment approach are critical for optimizing patient outcomes. Further research is needed to elucidate the mechanisms underlying such rare metastases and to explore potential treatment advancements.
TEACHING POINTS
Metastases to the pineal gland are uncommon, with the majority originating from primary tumors such as lung, breast, and gastrointestinal cancers. Melanoma metastasis to this region is particularly rare, with few cases reported in the literature.
On MRI, melanoma metastases often appear hyperintense on T1-weighted images, a feature attributed to the paramagnetic properties of melanin. This imaging characteristic can aid in differentiating melanoma from other pineal region tumors.
FDG-PET/CT can reveal increased metabolic activity in pineal lesions, consistent with metastatic melanoma. Additionally, this imaging modality assists in identifying other sites of metastasis, contributing to comprehensive staging.
MCQs
-
Which of the following imaging features is most suggestive of metastatic melanoma in the pineal gland?
non-enhancing cystic lesion with peripheral calcifications
Well-circumscribed, intensely enhancing mass with T1 hyperintensity and FDG avidity
T2 hyperintense lesion with homogeneous enhancement and restricted diffusion
Hypodense lesion on CT with no enhancement
Answer Key: b
-
What is the most common clinical presentation of metastatic melanoma to the pineal gland?
Seizures and focal neurological deficits
Progressive headaches, nausea, vomiting, and signs of obstructive hydrocephalus
Visual disturbances and auditory hallucinations
Sudden onset of hemiparesis and aphasia
Answer Key: b
-
Which imaging modality is most useful for assessing metabolic activity in suspected metastatic melanoma?
Non-contrast CT
Magnetic resonance spectroscopy
FDG-PET
SWI
Answer Key: c
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Melanoma brain metastasis presentation, treatment, and outcomes in the age of targeted and immunotherapies. Cancer. 2021;127:2062-73.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial metastases to the pineal region. Report of three cases. J Neurosurg Sci. 1991;35:55-7.
- [Google Scholar]
- Pineal gland metastasis from poorly differentiated carcinoma of unknown primary origin. Front Endocrinol (Lausanne). 2020;11:597773.
- [CrossRef] [PubMed] [Google Scholar]







