Translate this page into:
Solitary rectal diverticulum: A rare entity mimicking contained perforation – Imaging and surgical correlation
*Corresponding author: Rachita Khot, Department of Radiology, University of Virginia Health, Charlottesville, United States. rk9j@uvahealth.org
-
Received: ,
Accepted: ,
How to cite this article: Tulenko KG, Epstein SH, Kurpiel BR, Khot R. Solitary rectal diverticulum: A rare entity mimicking contained perforation – Imaging and surgical correlation. Case Rep Clin Radiol. doi: 10.25259/CRCR_126_2024
Abstract
Solitary rectal diverticulum is a rare entity, often asymptomatic and discovered incidentally. It may arise from congenital anomalies or acquired causes, including prior surgical interventions. Despite its rarity, complications such as diverticulitis, abscess formation, and fistulas can occur, mimicking more severe conditions such as perforation or perforated mass. Accurate diagnosis relies on precise imaging and pathology. Recognizing its distinct features is crucial for proper management, optimizing patient outcomes, and preventing misdiagnoses that could lead to inappropriate treatment strategies.
Keywords
Computed tomography
Diverticulitis
Magnetic resonance imaging
Rectal diverticulum
Solitary
INTRODUCTION
Rectal diverticula are rare anomalies, in contrast to the more common colonic diverticula. These diverticula have been reported in only a few cases. They do not show a typical age predilection but appear to affect men more frequently, with a ratio of 3:1.[1] Usually asymptomatic, rectal diverticula are typically discovered incidentally during imaging or endoscopic procedures. However, they can become symptomatic when filled with stool or inflamed, leading to various symptoms such as rectal pain, discomfort, and hematochezia. Like colonic diverticula, rectal diverticula can also result in complications such as diverticulitis, abscesses, fistulas, and perforation.[2,3]
CASE REPORT
A 45-year-old female with no known prior medical history presented to the Emergency Department with a 2 day history of hematochezia. She also reported a 4 year history of left lower quadrant and suprapubic pain, dysuria, difficulty urinating, back pain, numbness around the anus, and pain with defecation. She had no previous surgical history, but a colonoscopy performed 2 years ago in a different country revealed possible rectocolitis and multiple polyps. On physical examination, her abdomen was diffusely tender to palpation. Laboratory tests revealed a leukocytosis of 13 × 109/L, and her stool tested positive for guaiac.
On imaging, the computed tomography (CT) of the abdomen and pelvis with contrast in the sagittal plane showed a 15.4 × 13.6 × 8.2 cm thick-walled collection filled with stool and air within the pelvis [Figure 1a, arrow]. The wall is ill-defined and low density due to edema [Figure 1a]. CT coronal image demonstrates the communication of this heterogeneous collection with the rectum [Figure 1b]. The rectum is displaced laterally to the right and the urinary bladder is positioned superiorly. Magnetic resonance imaging (MRI) in the sagittal plane shows a large cavity containing a combination of air, fluid, and stool, and confirming the communication of the outpouching with the rectum [Figure 2a]. Coronal contrast-enhanced MRI shows intense enhancement of the thickened wall and mucosa, thus ruling out the possibility of contained perforation. There is stranding and enhancement in the perirectal fat indicating inflammation [Figure 2b]. No associated mass was noted. Axial diffusion-weighted image with high b value through the pelvis did not show restricted diffusion [Figure 2c and d]. The findings were confirmed on surgical pathology [Figure 3].
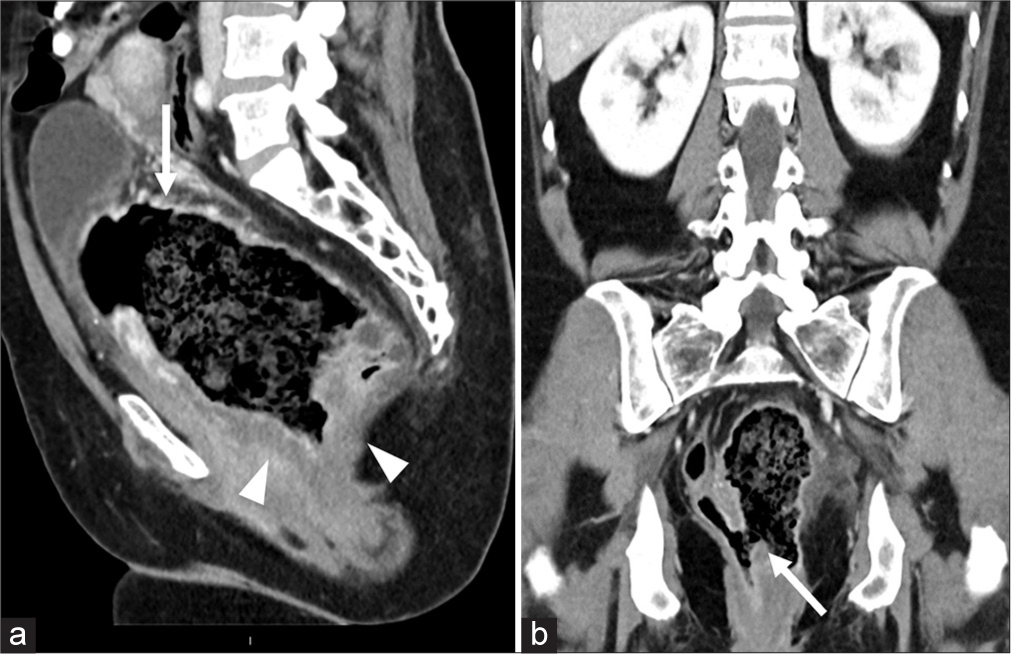
- Contrast enhanced computed tomography (a) sagittal image of the pelvis demonstrates a large central collection of air and fecal material (white arrow) with wall thickening (white arrowheads). (b) The coronal show communication of the central collection with the rectum (white arrow). The rectum is filled with air and is displaced laterally to the right.
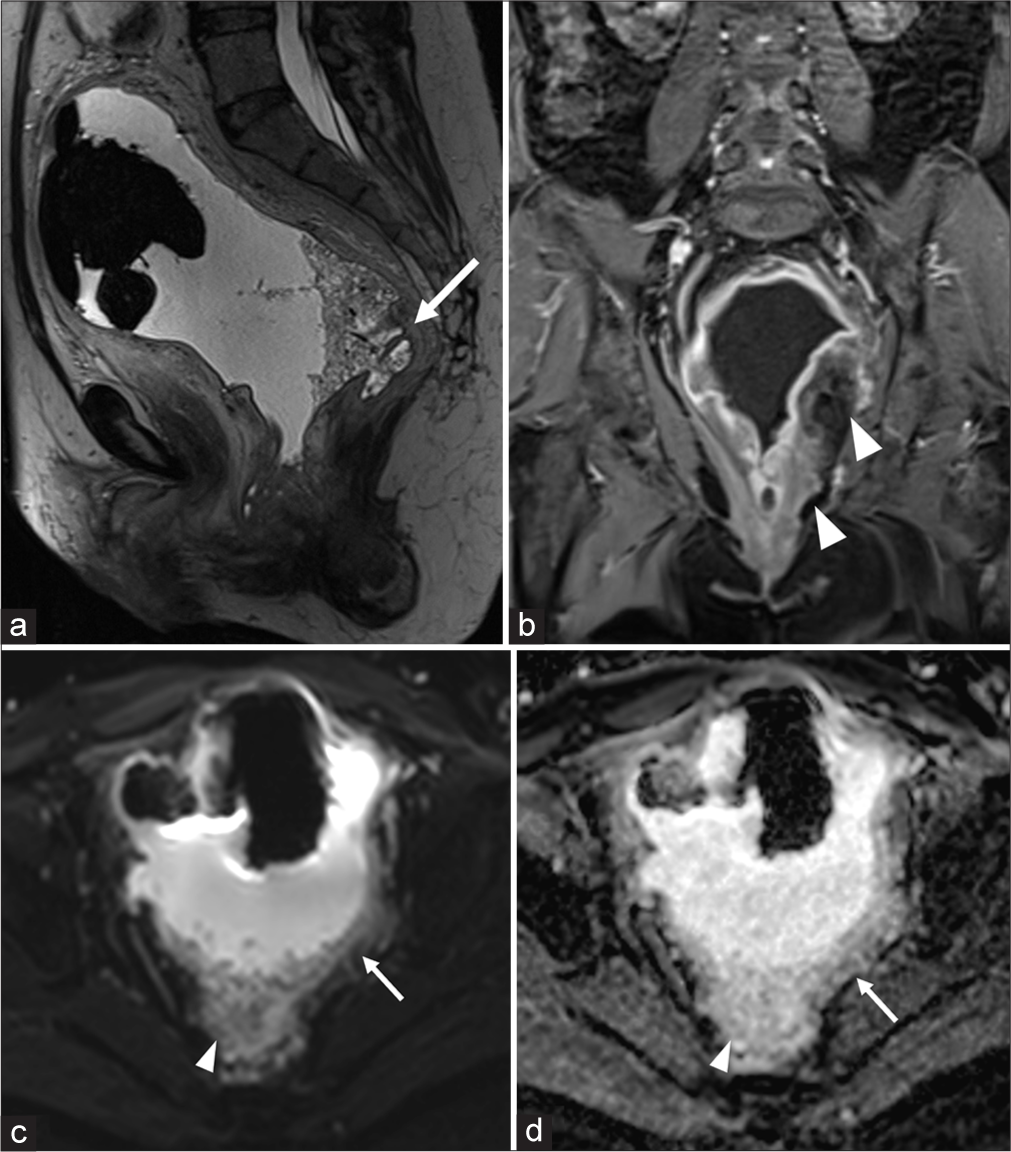
- Magnetic resonance imaging (a) sagittal T2WI image of the pelvis demonstrates the large diverticular cavity communicating with the rectum (white arrow). (b) Coronal contrast enhanced T1WI shows enhancing wall and mucosa with intense peri-diverticular inflammation (white arrowheads). (c and d) Diffusion-weighted image with b value-1000 and apparent diffusion coefficient show no restriction in and around the diverticulum (white arrows). The white arrowheads point to fecal material within the diverticulum.
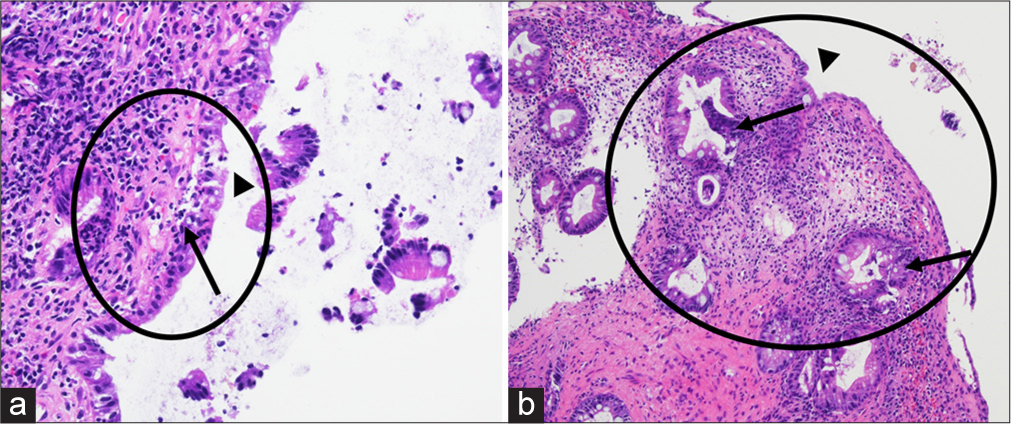
- On histology with hematoxylin and eosin staining at (a) 200x and (b) 100x magnification, the colorectal mucosa shows ulceration (black arrowheads) and granulation tissue (oval demarcated area) due to reactive changes including hyperplastic changes and crypt distortion (black arrows). Abundant acute inflammation is present in the form of scattered neutrophils throughout the specimen.
The patient subsequently underwent flexible sigmoidoscopy to assess the presence and extent of the diverticulum and to obtain tissue samples for pathologic diagnosis. Given the findings, the patient underwent a complete proctectomy with bilateral ureteral stent placement, total hysterectomy with bilateral salpingectomy, and posterior vaginal wall repair. Intraoperatively, significant inflammation was noted, causing adhesions and damage to surrounding structures. The patient’s post-operative recovery was uneventful, with no reported complications.
DISCUSSION
Rectal diverticula are true diverticula. A true diverticulum contains all the bowel layers, the muscularis, submucosa, and mucosa. Whereas a false or pseudodiverticulum consists of submucosa and mucosal layer.[2,3] The rarity in rectal diverticula formation can be attributed to the continuous layer of longitudinal and circular muscle fibers surrounding the rectal walls, creating a highly resistant wall against diverticula formation.[3,4] There is also decreased peristaltic activity and decreased internal pressure exerted on the walls of the rectum by feces compared to the colon. When rectal diverticulum does arise, it is often due to focal weakness of the rectal wall from either congenital or acquired causes. Factors contributing to their development include chronic constipation, weakening of the rectal wall, trauma or infection-related muscle atrophy, and surgical complications.[4] Procedures like stapled trans-anal rectal resection have also been documented to rectal diverticulum formation.[5] These diverticula occur along the lateral wall of the longitudinal muscle layer due to its thinner structure. The thinning causes weakness in the structural support of the rectal wall and thus is more susceptible to diverticula formation.[3]
Historically, barium enema was used to characterize rectal diverticula, but currently cross-sectional imaging is more commonly used.[6] In our case, CT and MRI imaging allowed for a comprehensive assessment of the rectal wall and facilitated the evaluation for complications or an underlying mass. Flexible sigmoidoscopy was necessary to further characterize the presence and extent of the diverticula. This sigmoidoscopy provided direct visualization of the outpouchings and allowed for tissue sampling for pathologic diagnosis.
The diagnosis is typically made based on radiological imaging and histopathological correlation. In our case, the biopsy from the rectal diverticula during sigmoidoscopy showed active colitis with ulceration but no evidence of malignancy or dysplasia which was concordant with the imaging findings. Treatment usually involves observation unless complications arise, in which case surgical resection of the diverticulum is the mainstay of treatment. The prognosis is generally good, with a low risk of recurrence. Rarely, there is a risk of malignancy with one reported case of mucinous adenocarcinoma within a rectal diverticulum.[7]
DIFFERENTIAL DIAGNOSIS
Contained perforation
Perforated mass
Ruptured duplication cyst.
CONCLUSION
Solitary rectal diverticula are a rare condition that can result from congenital defects or acquired causes. While they are often asymptomatic and discovered incidentally however, they share similar complications with the much more common colonic diverticula, including diverticulitis, abscesses, fistulas, and perforation.
TEACHING POINTS
The presence of intact mucosa and muscularis propria suggests the absence of a perforation, as contained perforations are typically sealed by surrounding inflamed and thickened tissue.
The absence of diffusion restriction and focal lesions excluded malignancy or malignant stricture, supporting an accurate diagnosis and guiding appropriate management.
Significant inflammation, evidenced by fat stranding and post-contrast enhancement around the diverticulum are best assessed on contrast enhanced images.
MCQs
-
Which of the following imaging modality is most commonly used to identify and assess a solitary rectal diverticulum?
Ultrasound
CT
MRI
Fluoroscopy with barium enema
Answer Key: b
-
What is the primary surgical intervention for symptomatic solitary rectal diverticulum?
Diverticulectomy with rectal repair
Hemicolectomy
Rectal stenting
No intervention
Answer Key: a
-
Solitary rectal diverticulum is most likely to present with which of the following symptoms?
Severe lower gastrointestinal bleeding
Asymptomatic or mild pelvic pain
Diarrhea with malabsorption
Small bowel obstruction
Answer Key: b
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Isolated rectal diverticulum complicating with rectal prolapse and outlet obstruction: Case report. World J Gastroenterol. 2005;11:7697-9.
- [CrossRef] [PubMed] [Google Scholar]
- A case of solitary rectal diverticulum presenting with a large retrorectal abscess. Ann Med Surg (Lond). 2020;49:57-60.
- [CrossRef] [PubMed] [Google Scholar]
- Large rectal diverticulum in the setting of pelvic organ prolapse treated with robotic ventral mesh rectopexy: A case report. Transl Cancer Res. 2023;12:1049-53.
- [CrossRef] [PubMed] [Google Scholar]
- Iatrogenic rectal diverticulum with pelvic-floor dysfunction in patients after a procedure for a prolapsed hemorrhoid. Ann Coloproctol. 2014;30:50-3.
- [CrossRef] [PubMed] [Google Scholar]
- Giant colonic diverticulum: Radiographic and MDCT characteristics. Insights Imaging. 2015;6:659-64.
- [CrossRef] [PubMed] [Google Scholar]
- A case of a mucinous adenocarcinoma arising from a rectal diverticulum. J Korean Soc Coloproctol. 2012;28:222-4.
- [CrossRef] [PubMed] [Google Scholar]







