Translate this page into:
Cracking the calcium code: Diagnosing hydroxyapatite deposition disease
*Corresponding author: Seetharaman Cannane, Department of Radiology, Kovai Medical Center and Hospitals, Coimbatore, Tamil Nadu, India. drcseetharaman@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gomathy B, Sugumar R, Cannane S. Cracking the calcium code: Diagnosing hydroxyapatite deposition disease. Case Rep Clin Radiol. doi: 10.25259/CRCR_67_2024
Abstract
Acute calcific periarthritis (ACP) of the great toe is a very rare presentation of hydroxyapatite deposition disease (HADD) in an unusual site, often causing acute pain, swelling, and reduced motion of the affected joint which may mimic trauma or infection. Diagnosis can be challenging, which often requires radiological imaging and the treatment is primarily conservative. In this case report, we wish to highlight the features of this rare entity in an unexpected site, that is, ACP of foot in a young female who presented with acute onset pain in the right great toe.
Keywords
Calcific periarthritis
Crystal arthropathy
HADD
INTRODUCTION
Acute calcific periarthritis (ACP) falls in the spectrum of hydroxyapatite crystal deposition disease (HADD) in which there is deposition of hydroxyapatite crystals in the periarticular soft tissues resulting in tendinitis and bursitis. The most commonly affected location is the shoulder, followed by hip, elbow, wrist, and knee.[1] Other less common locations include the ankle/foot and fingers.[1,2] It is usually asymptomatic and self-limiting. However, when there is a concomitant inflammatory process or when it develops in an unexpected site, it may mimic trauma, infection, or neoplasm. When symptomatic, HADD may present with pain, redness, swelling, and/or limited range of motion of the affected joints.[3] Since there are numerous clinical pathologies which can mimic HADD in unusual sites, the definitive diagnosis of this condition can be challenging.
CASE REPORT
A 23-year-old female presented with complaints of the right great toe swelling and pain for 3 weeks. No history of trauma or any other complaints were reported. The patient was evaluated with computed tomography (CT) and magnetic resonance imaging (MRI) of the right foot.
MRI revealed [Figure 1] small T2 hypointense focus in the periarticular region of the interphalangeal joint of the great toe along the medioplantar aspect. Significant adjacent periarticular soft-tissue inflammation was seen with post contrast enhancement. No abnormal fluid collection or diffusion restriction seen. CT revealed [Figure 2] amorphous and tiny foci of calcification adjacent to the interphalangeal joint of the right great toe. Dual-energy CT (DECT) analysis [Figure 2] revealed no evidence of monosodium urate crystal deposition. Based on these imaging findings, diagnosis of ACP (HADD) was made. The patient was conservatively treated with non-steroidal anti-inflammatory drugs (NSAIDs).

- (a and b) Axial gradient recalled echo (GRE), axial T2-weighted magnetic resonance imaging show tiny hypointense foci, corresponding to the calcific focus on computed tomography (red arrows). (c) Axial short-tau inversion recovery image shows inversion recovery hyperintensity around the interphalangeal joint of the great toe (green arrow). (d) Coronal T1-weighted post-contrast fat saturated image shows enhancing soft-tissue inflammatory changes around the interphalangeal joint of the great toe (green arrow).
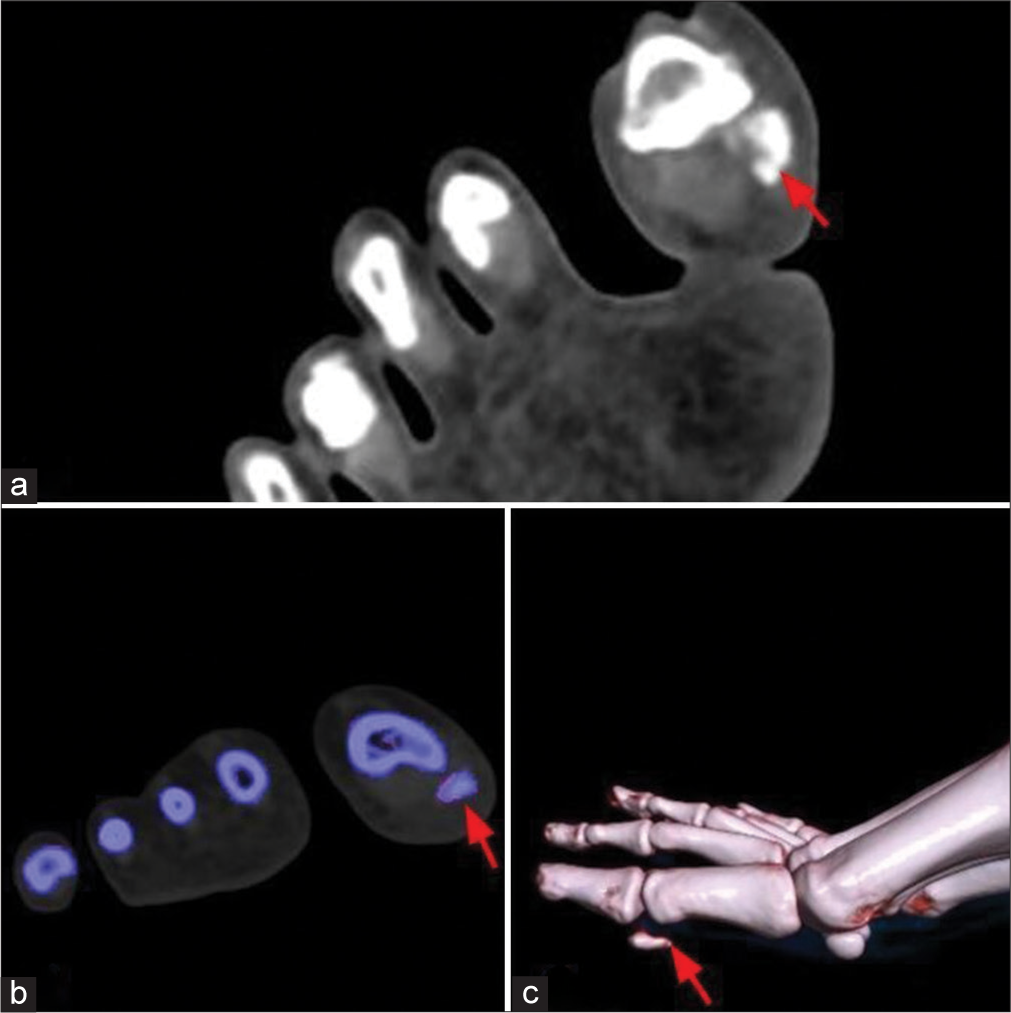
- (a) Computed tomography (CT) of the right foot shows abnormal irregular shaped and amorphous calcific foci in the periarticular region of the interphalangeal joint of the great toe along the medioplantar aspect (red arrow). (b) Dual-energy CT analysis does not show uric acid deposit (red arrow). (c) Volumetric reconstruction image of the right foot shows calcific focus adjacent to the interphalangeal joint of the great toe (red arrow).
DISCUSSION
Hydroxyapatite deposition disease (HADD) refers to a spectrum of abnormalities that includes calcific tendinitis, other periarticular hydroxyapatite deposition, and hydroxyapatite-induced arthritis. It is also known as calcific tendinosis, calcific periarthritis, peritendinitis calcarea, calcific peritendinitis, calcific bursitis, and hydroxyapatite rheumatism.[3] Calcium hydroxyapatite is the most common type of calcium in bone and pathologic calcifications.
Although the pathogenesis remains unknown, possible etiologies for calcium hydroxyapatite (CHA) deposition include local trauma, ischemia, and necrosis of tendons. It can be an idiopathic condition or it can be secondary to some other disease conditions like connective tissue disorders such as scleroderma, secondary hyperparathyroidism, or osteoarthritis. It has a higher prevalence in females compared to males.
HADD is a temporal process which changes with time and several classification schemes have been proposed. It includes the pre-calcific stage, followed by the calcific stage, during which the deposit is formed and then resorbed. In the post-calcific stage, tendon reconstitution occurs. As a result, symptoms may be chronic during the formative period and are often severe and acute during the resorption phase, needing hospitalization.[4] Patients might experience symptoms similar to those of a traumatic injury or illness during the acute phase of HADD, such as pain, edema, and limited range of motion in the affected area.
Plain radiography is the initial modality for evaluation of HADD, as it is sensitive for the detection of calcifications. It can differentiate HADD from mature ossifications which will show a distinct cortical or trabecular bone pattern, or foreign bodies that will have sharp/geometric borders.[5] HADD typically presents as well-defined, homogeneous, and amorphous calcifications during its formative phase, which is asymptomatic. On the other hand, it appears poorly defined, inhomogeneous, and fluffy during the resorption and acute symptomatic phase.
A comprehensive picture of hydroxyapatite deposits can be obtained through CT, which elucidates their location, size, and shape. It is the modality of choice for identifying calcific deposits. In CT scans, these deposits can be seen as high density zones within soft tissues, often associated with inflammation around them, resulting in increased attenuation. Progressed HADD stages might show hydroxyapatite deposits accompanied by bone erosion or cortical irregularities.[6] In addition, HADD can have a characteristic “comet tail” appearance on CT, which results from the longitudinal orientation of calcified deposits along tendons such that they seem to taper away from a site of bone involvement.[3]
Ultrasonography (US) is also a helpful tool for assessing HADD. Typically, calcifications appear hyperechoic with or without acoustic shadowing. Sonographically, HADD is characterized by well-defined, arc-shaped, and hyperechoic foci with posterior acoustic shadowing and no significant vascularity in the formative phase.[7] Calcifications seem ill-defined, non-arc shaped (fragmented, punctate, cystic, or nodular), less homogeneously hyperechoic, and have less distinct posterior acoustic shadowing during the symptomatic resorptive phase. Symptomatic resorptive HADD will also demonstrate vascularity on color Doppler images.[3] Calcifications associated with HADD are vascular in contrast to calcifications associated with degenerative non-HADD calcifications which occur in non-viable and devascularized tendons, typically at a tendon insertion or the edge of a tendon tear.[8]
MRI images show hydroxyapatite deposits as regions of low-signal intensity on both T1- and T2-weighted images, attributable to the high mineral content of hydroxyapatite. MRI also reveals inflammation signs within the surrounding soft tissues, such as edema and increased signal intensity on T2-weighted images. The characteristic “arc and ring” pattern of the deposits can occasionally be seen on MRI; this pattern shows a high-signal intensity rim of inflamed tissue surrounding a low-signal intensity core of hydroxyapatite crystals.[3] This pattern serves as a useful diagnostic marker in MRI of potential HADD patients. Gradient-recalled echo (GRE) sequences can show hydroxyapatite deposits as areas of signal voids or hypointensity due to the magnetic susceptibility effects of the calcified material. This is especially helpful in the early stages of HADD, when there may not be much hydroxyapatite deposition and it may be more difficult to find it using conventional imaging methods.[6]
CT or radiography correlation can be used to confirm the diagnosis of HADD when it is suspected but not yet proven, especially in cases when the condition presents in an unusual way or in an uncommon site.
The primary differential diagnosis for calcific periarthritis is gout. Gout typically presents as a monoarticular condition, commonly involving the first metatarsophalangeal joint. Radiographically, it displays well-defined “punched-out” erosions with sclerotic margins and overhanging edges (known as rat bite erosions). Periarticular soft-tissue swelling occurs due to monosodium urate crystal deposition around the joints. DECT aids in differentiation between uric acid crystals seen in gout and calcific deposits in calcific periarthritis.
When HADD presents acutely in the resorptive phase, it is usually a self-limited process and frequently resolves spontaneously within 2–3 weeks.[9] During the symptomatic phase, the primary management is conservative with NSAIDs and physiotherapy. A number of other non-invasive treatment modalities including treatment with acetic acid iontophoresis, ultrasound therapy, and extracorporeal shock wave therapy have also been described.[10] Modified US-guided fine-needle technique for calcific tendinitis (i.e., barbotage) which can be performed by the radiologist appears to be an effective therapy and was less aggressive as described in detail by Aina et al.[11] When conservative treatment approaches are ineffective, surgical excision of calcifications may be the next appropriate step.
DIFFERENTIALS DIAGNOSIS
Common pathologies which can mimic HADD include crystal arthropathies, septic arthritis, tenosynovitis, and erosive osteoarthritis [Table 1]:
| Crystal arthropathies • Gout • Pseudogout |
• Monoarticular, classically affecting the first metatarsophalangeal joint. • Well-defined “punched-out” erosions with sclerotic margins in a marginal and juxta-articular location and overhanging edges (rat bite erosions). • Periarticular soft-tissue swelling due to crystal deposition in tophi around the joints. • DECT - High attenuation crystal deposition corresponding to monosodium urate on dual-energy (Green) • Chondrocalcinosis is usually seen in CPPD disease or pseudogout. |
| • Septic arthritis | • Plain radiograph - Joint effusion, juxta-articular osteoporosis, narrowing of the joint space due to cartilage destruction, subchondral bone destruction on both sides of a joint • Ultrasound - Joint effusion with echogenic debris. Increased peri-synovial vascularity. • MRI - Synovial enhancement on post contrast images with pericapsular edema and enhancement. |
| • Tenosynovitis | • Plain radiograph - calcification of synovial membrane and adjacent bone periosteal reaction • Ultrasound - Thickening of the synovial sheath with or without increased vascularity which can extend into the tendon sheath, and peritendinous subcutaneous edema. • MRI - Tendon sheath thickening and peritendinous subcutaneous contrast enhancement in tenosynovitis. |
| • Erosive osteoarthritis | • Erosive osteoarthritis predominantly involves the interphalangeal joints (proximal and distal) and first carpometacarpal joints of the hands. • Subchondral erosions and classic central necrosis (Gull-wing appearance) seen in erosive osteoarthritis. • Absence of marginal erosions, soft tissue swelling and osteopenia. |
DECT: Dual-energy computed tomography, CPPD: Calcium pyrophosphate deposition, MRI: Magnetic resonance imaging
CONCLUSION
Numerous conditions are characterized by the deposition of hydroxyapatite crystals. It may be confused for other crystal arthropathies, inflammation, trauma, or infection due to its associated inflammatory response or its propensity for cortical erosion. Familiarity with the range of HADD presentations aside from their typical locations is critical for effective diagnosis. Early diagnosis can avoid costly work-up and interventions.
TEACHING POINTS
Characteristic amorphous calcific deposition, thickened tendon/ligament, and adjacent soft-tissue inflammation are the key imaging features of HADD
Combined with the patient’s clinical presentation, history and radiological imaging findings, aid in the accurate diagnosis of HADD and differentiation from other conditions.
MCQs
-
Which one of the following sentences is false regarding HADD?
It is due to deposition of calcium hydroxyapatite.
Most common affected location is the shoulder.
DECT cannot differentiate between gout and HADD.
There are 3 stages of HADD – pre-calcific, calcific and post-calcific stage.
Answer Key: c
-
What is the standard modality for diagnosis of HADD?
Ultrasound
CT
Plain radiograph
MRI
Answer Key: b
-
Which one of the following is false regarding HADD?
Gout is one of the close differential diagnoses.
Primary management is conservative with NSAIDs
Punched-out erosions with overhanging edges are characteristic of HADD.
“Arc and ring” is a characteristic MRI pattern of HADD.
Answer Key: c
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Calcium hydroxyapatite deposition disease. Radiographics. 1990;10:1031-48.
- [CrossRef] [PubMed] [Google Scholar]
- Acute calcific periarthritis of proximal interphalangeal joint in a professional golfer's hand. J Korean Med Sci. 2004;19:904-6.
- [CrossRef] [PubMed] [Google Scholar]
- Calcium hydroxyapatite deposition disease: Imaging features and presentations mimicking other pathologies. Eur J Radiol. 2019;120:108653.
- [CrossRef] [PubMed] [Google Scholar]
- Calcifying tendinitis. Baillieres Clin Rheumatol. 1989;3:567-81.
- [CrossRef] [PubMed] [Google Scholar]
- Radiological identification and analysis of soft tissue musculoskeletal calcifications. Insights Imaging. 2018;9:477-92.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxyapatite deposition disease: A comprehensive review of pathogenesis, radiological findings, and treatment strategies. Diagnostics. 2023;13:2678.
- [CrossRef] [PubMed] [Google Scholar]
- Calcific tendinitis of the rotator cuff: State of the art in diagnosis and treatment. J Orthop Traumatol. 2016;17:7-14.
- [CrossRef] [PubMed] [Google Scholar]
- Calcific tendinitis of the rotator cuff. World J Orthop. 2016;7:55-60.
- [CrossRef] [PubMed] [Google Scholar]
- Methods and results in the treatment of 2,580 painful shoulders: With special reference to calcific tendinitis and the frozen shoulder. Am J Surg. 1958;95:527-44.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound therapy for calcific tendinitis of the shoulder. N Engl J Med. 1999;340:1533-8.
- [CrossRef] [PubMed] [Google Scholar]
- Calcific shoulder tendinitis: Treatment with modified US-guided fine-needle technique. Radiology. 2001;221:455-61.
- [CrossRef] [PubMed] [Google Scholar]








