Translate this page into:
Where does it come from? A large pelvi-abdominal mass with points to ponder
*Corresponding author: Meera Kochuveetil Vijayakumar, Department of Radiodiagnosis, Government Medical College Konni, Pathanamthitta, Kerala, India. meerakvdr@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kochuveetil Vijayakumar M, Zachariah SA, Mathew J. Where does it come from? A large pelvi-abdominal mass with points to ponder. Case Rep Clin Radiol. doi: 10.25259/CRCR_125_2023
Abstract
Ureteral leiomyosarcoma is a very rare aggressive retroperitoneal malignancy with limited possibility of an accurate pre-operative diagnosis. Incidence of ureter leiomyosarcoma is extremely rare. Hence, there is paucity of reported cases in the literature. We present a case of a 60-year-old postmenopausal woman presenting with abdominal distension and discomfort. This was initially reported as a malignancy arising from left ovary, as the lesion had apparent convergence toward left adnexal region and the left ovary was not seen separately. In addition, there was moderate hydronephrosis of the left kidney and left ureter was not separately made out from the lesion. Leiomyosarcoma should be considered as a differential diagnosis in cases of ureter stricture and retroperitoneal tumors causing ureteric obstruction.
Keywords
Leiomyosarcoma
Ureter
Neoplasm
Retroperitoneum
INTRODUCTION
Leiomyosarcoma of the ureter is an exceedingly rare malignant urinary tract neoplasm less often reported in the literature.[1,2] Majority of ureter neoplasms are transitional cell carcinoma. Among the various sarcomas reported in the urinary tract, leiomyosarcoma runs an aggressive course with invasion of adjacent organs. The lesion often metastasizes to liver, lungs, regional lymph nodes, and mesentery.[2-5] Most cases of ureter leiomyosarcoma present as obstruction in the distal ureter. As there is no involvement of the mucosa, hematuria is an extremely rare presentation.[6]
CASE REPORT
A 60-year-old postmenopausal woman presented with history of loss of appetite and abdominal distension of 6 months duration. She had diabetes and was on oral hypoglycemic agents. On clinical examination, there was a vague, hard and non-tender mass in the left side of abdomen and pelvis which was moving with respiration. Ultrasonography performed in our department revealed a moderate left hydronephrosis with thinning of the left renal parenchyma. Left ureter was not seen separately. A large lobulated heterogeneously hypoechoic lesion was noted on the left side of pelvis extending to abdomen. Ovaries were not separately seen [Figure 1a and b]. The patient underwent a contrast-enhanced computed tomography (CT) examination, revealing a well-defined, lobulated soft-tissue density lesion measuring 16 × 12.5 × 20 cm on the left side of the abdomen and pelvis. It exhibited heterogeneous post-contrast enhancement with non-enhancing areas within, likely representing foci of necrosis [Figure 2a and b Arrow].

- Sixty-year-old post woman presenting with abdominal distention and discomfort. Initial ultrasound images (a) shows moderate left hydronephrosis with thinning of renal parenchyma and (b) shows a large lobulated hypoechoic lesion in the left side of abdomen and pelvis.
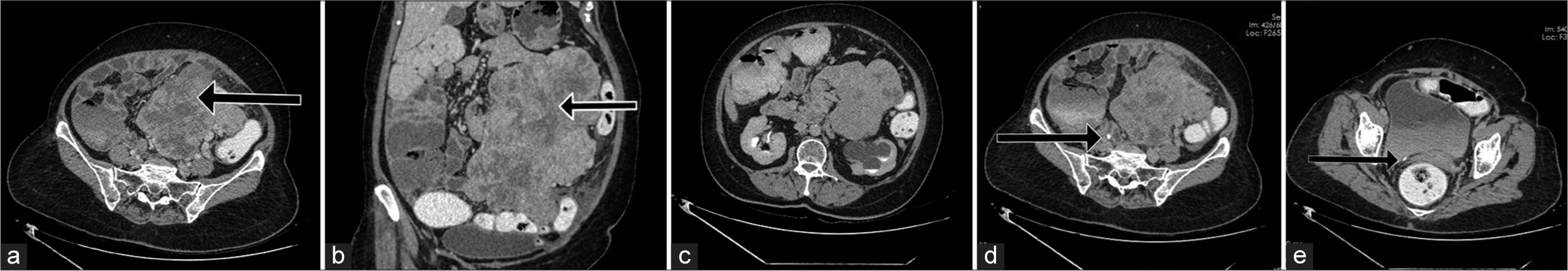
- Sixty-year-old post woman presenting with abdominal distention and discomfort. (a) Contrast-enhanced computed tomography (CECT) image in venous phase axial section shows a heterogeneously enhancing soft-tissue density lesion in the left side of abdomen and pelvis (black arrow). (b) CECT image in venous phase coronal reformatted section shows non-enhancing areas within the lesion, suggestive of necrosis (black arrow). (c) CECT image in delayed phase shows delayed contrast excretion from the left hydronephrotic kidney. Left ureter not separately seen from the lesion. (d) depicts absence of contrast opacification in the left ureter in axial sections in delayed phase. Opacifed right ureter is seen (black arrow). (e) Section through lower pelvis shows no contrast filling in the left ureter. Arrow represents normally opacified distal right ureter.
There was delayed contrast excretion of the left kidney in 1 h delayed images [Figure 2c]. Left ureter was not separately seen from the lesion. The left ureter did not show contrast opacification in 3 min and 1 h delayed images while right ureter demonstrated normal opacification [Figure 2d and e].
Due to the apparent convergence of the lesion toward the left adnexa and the inability to visualize the left ovary separately, the lesion was reported as a malignant solid neoplasm likely originating from the left ovary. In addition, since the left ureter did not exhibit contrast opacification, infiltration of the left ureter by the mass lesion was suspected, resulting in upstream hydronephrosis of the left kidney. Our imaging differentials included retroperitoneal sarcoma and extraintestinal gastrointestinal stromal tumor (GIST). Trucut biopsy of the lesion was performed under ultrasound guidance, which revealed malignant spindle cell neoplasm. The patient underwent complete excision of the tumor, left nephroureterectomy, and bladder cuff excision. Operatively, there was a large multilobulated soft-tissue tumor arising from the retroperitoneum infiltrating the whole of the left ureter with non-infiltrative adhesions to left colon, mesentery, left renal pelvis, and pancreatic tail. No skip lesions were seen. No peritoneal metastases were identified. The operative specimen was sent for histopathological examination.
Gross specimen had a bosselated surface with congested blood vessels and adipose tissue. Cut surface appeared whitish whorled with focal yellowish areas [Figure 3a]. Lymphovascular invasion was seen with tumor reaching up to and involving the circumferential resected margins. Proximal and distal resected margins of ureter were free of neoplasm.
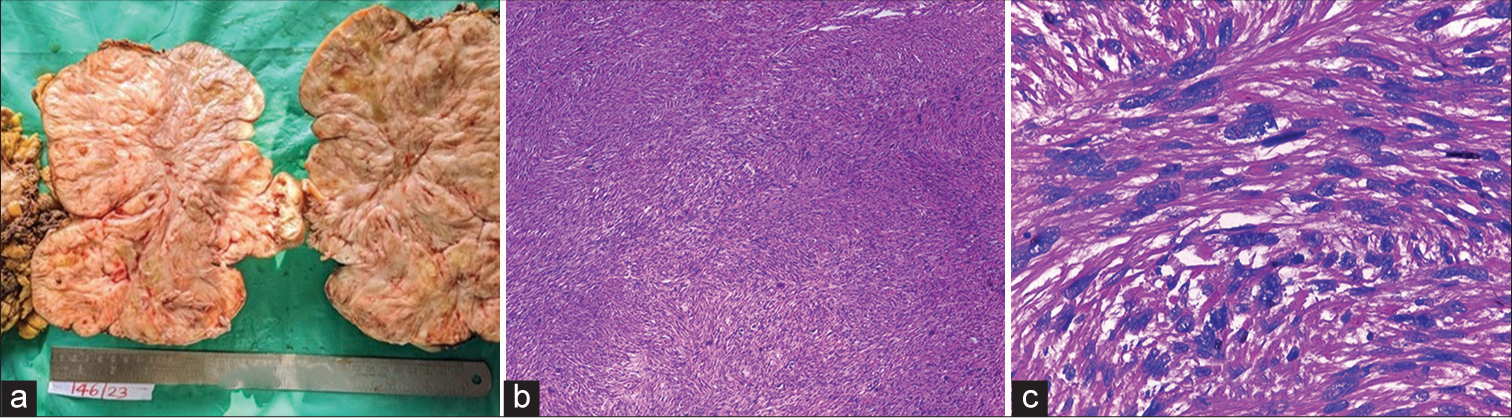
- Sixty-year-old post woman presenting with abdominal distention and discomfort. (a) Gross pathology specimen cut surface appeared whitish whorled with focal yellowish areas, (b) microscopy demonstrates infiltrating neoplasm arising from the wall of ureter, composed of cells arranged interlacing fascicles, bundles and focal storiform pattern (Hematoxylin & Eosin 10x), and (c) magnified view shows individual cells that are spindle shaped with moderate eosinophilic cytoplasm and moderate to focal marked nuclear pleomorphism-findings in favor of malignant spindle cell neoplasm (Hematoxylin & Eosin 40x).
Microscopy revealed infiltrating neoplasm arising from the wall of ureter, composed of cells arranged interlacing fascicles, bundles, and focal storiform pattern. Individual cells were spindle shaped with moderate eosinophilic cytoplasm and moderate to focal marked nuclear pleomorphism. These findings were in favor of malignant spindle cell neoplasm [Figure 3b and c].
Immunohistochemistry was performed in our case, which yielded positive results for Desmin and Smooth Muscle Actin (SMA). Following resection of the retroperitoneal tumor along with the left kidney, left ureter, and bladder cuff, the patient was initiated on chemotherapy.
DISCUSSION
Retroperitoneal masses that do not originate from organs such as the kidneys, adrenal glands, pancreas, or bowel loops are classified as primary retroperitoneal tumors.[5] Four useful radiological signs have been described as diagnostic clues for identification of organ of origin in retroperitoneal tumors. They are (1) beak sign, (2) phantom (invisible) organ sign, (3) embedded organ sign, and (4) prominent feeding artery sign.[7]
In beak sign, when a mass distorts the edge of an adjacent organ into a “beak,” it is likely that the mass arises from that organ. Phantom organ sign refers to the inability to see a small organ when a large mass arises from it. Positive embedded organ sign means that the mass appears to be embedded in its organ of origin. An adjacent plastic organ will only be compressed to a crescent by the retroperitoneal mass (negative embedded organ sign). Prominent feeding artery sign refers to the hypervascular mass supplied by large prominent feeding vessels that are well visualized by CT and magnetic resonance imaging, providing an important clue to the organ of origin.[7]
When no definite diagnostic clues to suggest the organ of origin, then the possibility of primary retroperitoneal lesion is to be considered.[7]
Leiomyosarcoma is a rare aggressive malignant soft-tissue neoplasm with dismal prognosis.[8] There is paucity of reported cases in the literature due to rarity of presentation. Imaging features depend on the organ of origin. The presence of calcification and fat is uncommon. The lesions often show heterogenous appearance with areas of necrosis as in our case. Ureteral leiomyosarcoma is more common in middle-aged women than in men. Positivity of Desmin and SMA is helpful to make the final diagnosis. Negative myoglobin, cytokeratin, and S-100 help to rule out rhabdomyosarcoma, sarcomatoid carcinoma, and melanoma, respectively.[9,10]
DIFFERENTIAL DIAGNOSES
Extraintestinal GIST
Retroperitoneal sarcoma
Malignant peripheral nerve sheath tumors
Inflammatory myofibroblastic tumor
CONCLUSION
Ureteral leiomyosarcoma should be considered in the differential diagnosis of retroperitoneal masses especially when there is involvement of the urinary tract. It is important to differentiate it from other soft-tissue sarcomas such as rhabdomyosarcoma and other spindle cell neoplasms. Immunohistochemistry plays a crucial role in diagnosis. Management is nephroureterectomy with bladder cuff resection followed by chemotherapy.
TEACHING POINTS
Careful evaluation for the CT signs of retroperitoneal tumors is important when the organ of origin is doubtful in cases of large lesions.
When encountered with large retroperitoneal masses with concurrent ureteric obstruction, leiomyosarcoma of the ureter can be considered as a differential diagnosis.
MCQs
-
Common CT signs of retroperitoneal tumors include
Beak sign
Embedded organ sign
Phantom organ sign
All of the above
Answer Key: d
-
Positivity of Desmin and SMA suggests the tissue of origin as
Smooth muscle
Skeletal muscle
Cardiac muscle
Bone
Answer Key: a
-
Most common retroperitoneal sarcoma is
Leiomyosarcoma
Liposarcoma
Rhabdomyosarcoma
Fibrosarcoma
Answer Key: b
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Primary leiomyosarcoma of the middle ureter: A rare case report with literature review. Clin Case Rep. 2022;10:e05409.
- [CrossRef] [PubMed] [Google Scholar]
- Primary benign and malignant tumors of the ureter; a review of the literature and report of one benign and twelve malignant tumors. Am J Surg. 1956;91:237-71.
- [CrossRef] [PubMed] [Google Scholar]
- Practical approach to primary retroperitoneal masses in adults. Radiol Bras. 2018;51:391-400.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of primary retroperitoneal masses by computed tomography scan. Int J Med Sci Public Health. 2016;5:1423-9.
- [CrossRef] [Google Scholar]
- Primary retroperitoneal neoplasms: CT and MR imaging findings with anatomic and pathologic diagnostic clues. Radiographics. 2003;23:45-57.
- [CrossRef] [PubMed] [Google Scholar]
- Leimyosarcoma of ureter presenting as acute renal failure. Br J Urol. 1979;51:326.
- [CrossRef] [PubMed] [Google Scholar]
- Antibody specific to muscle actions in the diagnosis and classification of soft tissue tumors. Am J Pathol. 1988;130:205-5.
- [Google Scholar]
- Immunohistochemical detection of cytokeratin and epithelial membrane antigen in leiomyosarcoma: A systematic study of 100 cases. Pathol Int. 2000;50:7-14.
- [CrossRef] [PubMed] [Google Scholar]







