Translate this page into:
Sinking skin flap syndrome: A rare complication following a craniectomy
*Corresponding author: Dhruv Nihal Gandhi, Department of Internal Medicine, KJ Somaiya Medical College and Research Centre, Mumbai, Maharashtra, India. dhruvgandhi2610@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Joshi RV, Gandhi DN, Lathiya H, Thapar RB, Kamat R. Sinking skin flap syndrome: A rare complication following a craniectomy. Case Rep Clin Radiol. doi: 10.25259/CRCR_82_2024
Abstract
Sinking skin flap syndrome is a rare condition in which neurological deterioration is seen following a large craniectomy. Patients present with sensorimotor deficits and altered mental status which may be difficult to distinguish clinically from other causes of neurological deterioration in post-craniectomy patients. The radiologist’s role is critical in such cases for early detection and prompt initiation of treatment. We present a case of sunken skin flap syndrome in a 66-year-old man following a large craniectomy done for a stroke.
Keywords
Sinking skin flap syndrome
Syndrome of the trephined
Midline shift
Craniectomy complications
Intracranial hemorrhage
INTRODUCTION
Sinking skin flap syndrome (SSFS) or “syndrome of the trephined” or paradoxical hernia is a rare complication seen in around 10% of cases following a large craniectomy.[1,2] This is typically seen a few months to years after the surgery and presents with acute-onset symptoms of progressive neurological deterioration.[3] This condition is difficult to clinically distinguish from other causes of altered consciousness in post-craniectomy patients and is usually diagnosed radiologically.[4] Thus, radiologists need to be aware of this condition and its imaging findings. We present a 66-year-old man who underwent a large craniectomy for a stroke and presented 1 month later with neurological deterioration. On computerized tomography (CT) brain, he was diagnosed with SSFS.
CASE REPORT
A 66-year-old man presented to the casualty in October 2023, approximately 18 h after the onset of left-sided weakness and difficulty in speaking. He had a history of diabetes and hypertension, both controlled on medication, and a history of ischemic heart disease for which he had undergone a coronary artery bypass grafting 1 year back. On examination, he was found to have no power in both the left upper and lower limbs and his speech was slurred. The tone was moderately increased (3+) and the knee and elbow reflexes were brisk on the left side. Other general and systemic examinations were normal.
The patient was admitted to the intensive care unit (ICU) and a magnetic resonance imaging brain with angiography was done. It showed a large wedge-shaped acute infarction involving the right frontotemporoparietal lobes in the middle cerebral artery (MCA) distribution. Hemorrhagic changes were seen within the infarct showing blooming on susceptibility-weighted imaging sequence [Figure 1a]. The infarct showed restricted diffusion and a T2 hyperintense signal abnormality [Figure 1b]. There was an associated mass effect causing effacement of the ipsilateral sulci and lateral ventricle with midline shift to the left of approximately 7 mm [Figure 1b]. Magnetic resonance angiography revealed focal severe stenosis of the distal M1 segment of the right MCA with mild paucity of the cortical branches of the right MCA.
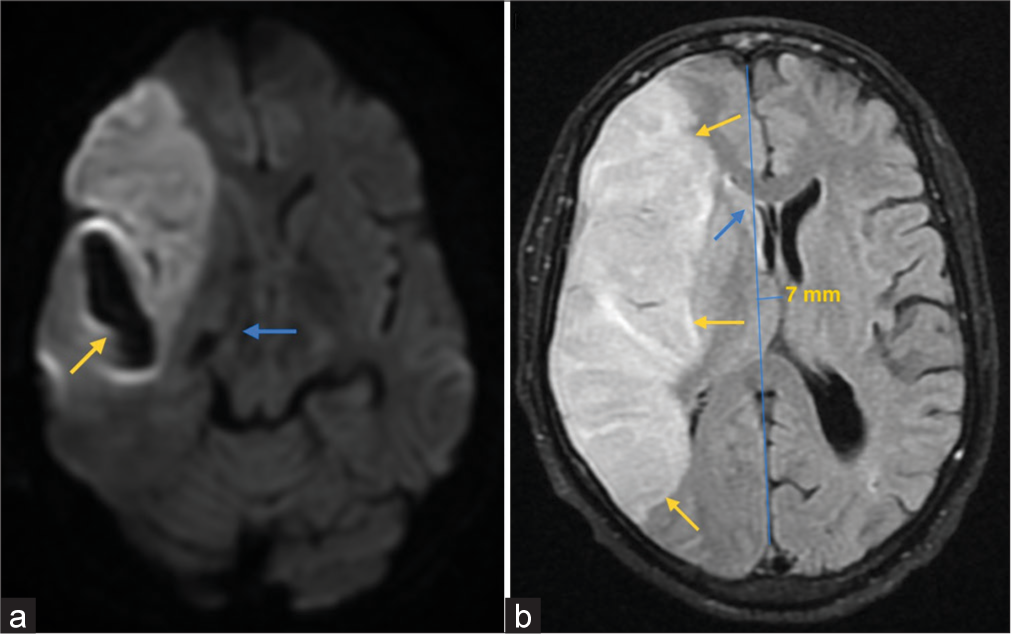
- (a) MRI Brain susceptibility weighted imaging (SWI) sequence showing an area of blooming involving the region of the right temporal infarction (yellow arrow), suggestive of the hemorrhagic changes within. (b) MRI Brain fluid-attenuated inversion recovery (FLAIR) sequence showing hyperintensities in right frontoparietal region (yellow arrows) with mass effect in the form of effacement of ipsilateral sulci and lateral ventricle (blue arrow). There is also mild distortion of the ipsilateral side of mid brain (blue arrow in a) and a midline shift of 7 mm towards left.
A right frontoparietal decompressive craniectomy was performed and the hemorrhage was evacuated. Post-surgical recovery was good, and the patient was discharged 10 days later. Follow-up with serial brain CT scans post-surgery was done. Five-week post-craniectomy, he suddenly became drowsy and was admitted to the ICU. On examination, he was found to be arousable. There were no raised intracranial pressure symptoms, and he was vitally stable.
A CT brain was performed which revealed a sunken flap over the right frontoparietal region and effacement of the right lateral ventricle and right cerebral sulci [Figure 2]. The volume loss of brain parenchyma due to the stroke may cause some indrawing of flap but in such scenarios, the midline shift is toward the same side as the craniotomy. However, in this case, the sunken craniotomy flap was clearly associated with mass effect causing effacement of ipsilateral sulci and lateral ventricle with midline shift to the contralateral side [Figure 2]. A 3D CT reconstruction of the craniectomy site is shown in Figure 3. He was diagnosed with sunken skin flap syndrome and managed conservatively by positioning in Trendelenburg’s position to raise the intracranial pressure and hydrating on intravenous saline. Cranioplasty was not performed. The patient gradually recovered on conservative therapy and was discharged home.
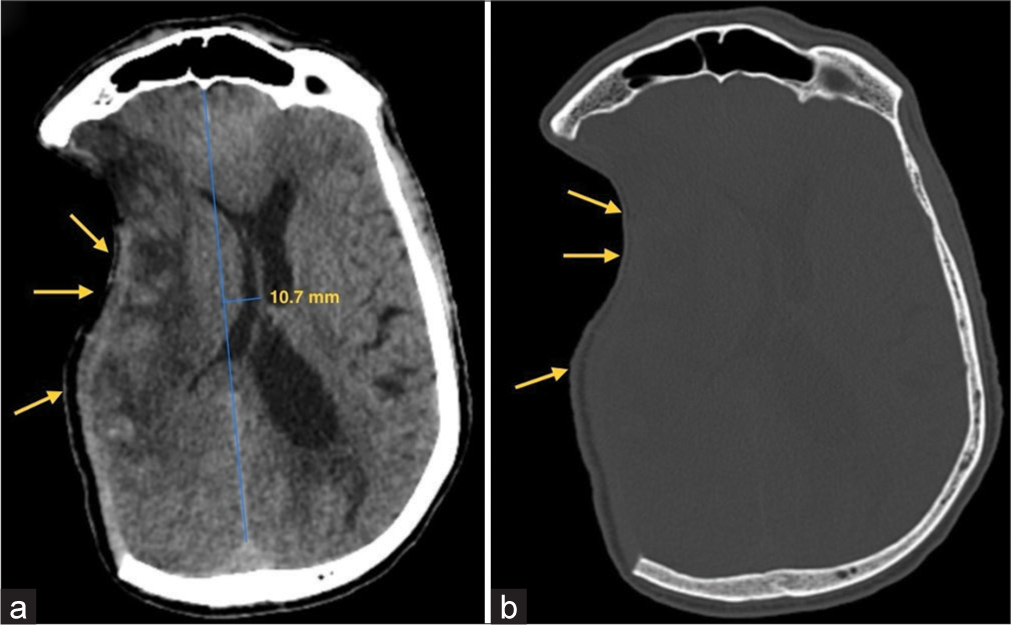
- (a) CT Brain axial section reveals ill-defined hypodensities involving right frontoparietal region consistent with large right middle cerebral artery (MCA) territory infarct with mass effect in form of effacement of the ipsilateral sulci (yellow arrows) and lateral ventricle and paradoxical midline shift of 10.7 mm away from the craniectomy site. (b) CT Brain bone window image shows right frontoparietal craniectomy defect with overlying indrawing of the skin flap (yellow arrows).
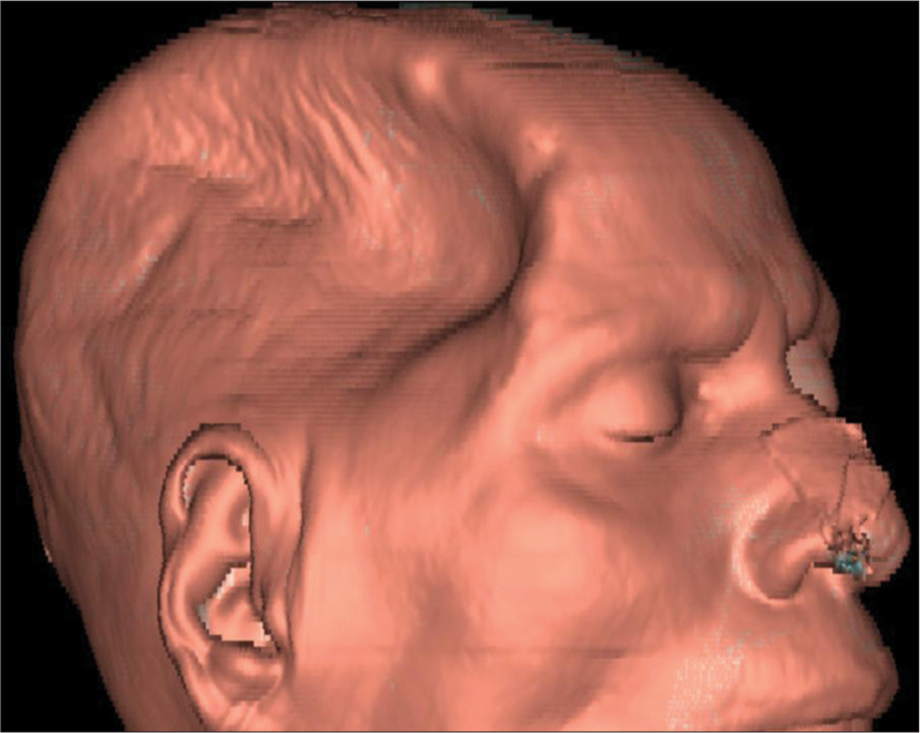
- 3D CT reconstruction showing a sunken skin flap over the craniectomy site in the right frontoparietal area.
DISCUSSION
SSFS or “syndrome of the trephined” or paradoxical hernia is an uncommon complication of a large craniectomy usually seen a few months after the procedure.[1,2] Ever since the first case was reported by Yamamura in 1977, several underlying pathologies leading to craniectomies have been found to be associated with this syndrome including traumatic brain injuries, ischemic strokes, and intracranial hematomas.[2]
Patients typically present with sudden onset or progressive neurological worsening with altered mental status, headache, seizures, weakness, fatigue, dizziness, and acute focal neurological deficits. On examination, a large skull defect is often found with an overlying depressed skin flap.[3,4]
From a radiological standpoint, the brain of a post-craniectomy patient can experience one of three scenarios: (1) The brain is at the same level as the craniectomy flap; (2) the brain is sunken (sunken flap sign); or (3) the brain has herniated extracranially. In the context of a sunken brain, a contralateral displacement of the midline may develop, resulting in a condition known as paradoxical herniation. Sometimes, SSFS can be seen in patients who do not have the sunken flap sign, implying that the name may be a misnomer.[5]
Several theories exist behind the pathophysiology of this syndrome. The older theory is that the sunken flap is caused by pulsing intracranial arterial or venous pressure movements. Another explanation suggests that the skull defect could cause a suction effect on cerebrospinal fluid (CSF) dynamics by distorting the dura, cortex, and venous return through scarring and direct pressure.[6] This pathophysiological mechanism is supported by the finding that neurological improvement post-cranioplasty was best seen in those patients who had large defects close to dural venous sinuses that allowed for the atmospheric pressure to compress the cortex and dural scarring to hamper venous return.[7] Neurological impairment has also been hypothesized to result from a negative gradient between atmospheric and intracranial pressure, which is exacerbated by CSF compartment alterations following CSF hypovolemia.[6] This theory is supported by the finding that patients of neurological disorders with raised intracranial pressure showed prolonged improvement when treated with cranioplasty as opposed to external decompression.[7] The hypothesis most relevant to our case is that the sunken skin flap and paradoxical herniation results from the atmospheric pressure being conveyed to the cranial cavity, which leads to flap involution and subsequent neurological deterioration.[6]
The initial management of SSFS involves supportive measures as well as collaborative management with neurosurgery. Supportive measures include putting the patient in Trendelenburg position which helps to restore the normal scalp curvature and has been found to be strongly related to neurological recovery.[8] Cases of Glasgow coma scale improvement and symptom resolution have been reported simply by putting the patient in Trendelenburg position. Intravenous fluids are also given to prevent hypotension as that can reduce cerebral perfusion and lower the intracranial pressure, thus worsening the herniation.[8]
Urgent surgical intervention is rarely necessary; nevertheless, in individuals who do not respond to conservative treatment, early cranioplasty is most helpful in reducing symptoms. Early cranioplasty is considered a life-saving measure to undertake when a midline shift is confirmed by CT and does result in better results for these individuals.[4] Another option from the surgical standpoint is to perform a scar tissue lysis procedure.[8] A recent study has demonstrated the use of vacuum-assisted closure therapy in severe sunken skin flap syndrome.[9]
CONCLUSION
Radiological investigations are important in the diagnosis of SSFS, emphasizing the importance of recognizing the imaging findings of this syndrome in post-craniectomy patients. Early identification and, when necessary, cranioplasty plays a crucial role in improving patient outcomes.
TEACHING POINTS
Sunken skin flap syndrome is a rare but life-threatening complication which can occur following a large craniectomy.
The visualization of the sunken flap on CT/MRI and the contralateral midline shift of the brain parenchyma are crucial imaging findings for the early detection and prompt treatment of this condition.
MCQs
-
Which of the following theories best explains the pathophysiology of SSFS?
Pulsing intracranial arterial or venous pressure changes
Skull defect causing suction effect on CSF dynamics by distorting the dura, cortex, and venous return, through scarring and direct pressure
CSF compartment alterations following CSF hypovolemia, causing the flap to sink
Atmospheric pressure being conveyed to the brain cavity, causing the scalp to inwardly involute over the cranial defect
Answer Key: d.
-
Which of the following are not consistent with the imaging findings of SSFS?
Sunken flap over the region of the craniectomy defect
Volume loss of brain parenchyma and indrawing of the flap
Effacement of the ipsilateral sulci and lateral ventricle
Ipsilateral midline shift (toward the same side as craniectomy)
Answer Key: d.
-
Which of the following may be used in the management of SSFS?
Placing the patient in Trendelenburg position
Hydration with intravenous fluids
Cranioplasty
All of the above
Answer Key: d.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Sinking skin flap syndrome, a rare complication of craniectomy. J Belg Soc Radiol. 2022;106:52.
- [CrossRef] [PubMed] [Google Scholar]
- Sinking skin flap syndrome: Phenomenon of neurological deterioration after decompressive craniectomy. Case Rep Med. 2018;2018:9805395.
- [CrossRef] [PubMed] [Google Scholar]
- Sinking skin flap syndrome in the multi-trauma patient: A paradoxical management to TBI post craniectomy. J Surg Case Rep. 2020;2020:rjaa172.
- [CrossRef] [PubMed] [Google Scholar]
- Sinking skin flap syndrome after decompressive hemicraniectomy in a patient with calvarial multiple myeloma who underwent a lumbar puncture: A case report. Cureus. 2022;14:e24458.
- [CrossRef] [PubMed] [Google Scholar]
- Sinking skin syndrome in a decompressive craniectomy series: Clinical and radiological features. Surg Neurol Int. 2022;13:422.
- [CrossRef] [PubMed] [Google Scholar]
- Sinking skin flap syndrome in a patient with bone resorption after cranioplasty and ventriculoperitoneal shunt placement: Illustrative case. J Neurosurg Case Lessons. 2021;2:CASE21359.
- [CrossRef] [PubMed] [Google Scholar]
- Motor and neurocognitive recovery in the syndrome of the trephined: A case report. Ann Phys Rehabil Med. 2015;58:183-5.
- [CrossRef] [PubMed] [Google Scholar]
- Novel temporary treatment for a severe case of syndrome of trephined. World Neurosurg. 2018;120:200-4.
- [CrossRef] [PubMed] [Google Scholar]







