Translate this page into:
Neurobrucellosis presenting as an infected epidermoid cyst in the cerebellum – A case report
*Corresponding author: Dipankar Pal, Department of Infectious Diseases, Christian Medical College, Vellore, Tamil Nadu, India. dipankarpal.2009@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pal D, Vanjare HA, Rupali P, Prasad KB. Neurobrucellosis presenting as an infected epidermoid cyst in the cerebellum – A case report. Case Rep Clin Radiol. doi: 10.25259/CRCR_25_2024
Abstract
Brucellosis may involve almost every organ of the human body. The central nervous system (CNS) is involved through hematogenous dissemination during acute septicemic brucellosis. CNS symptoms may manifest during the acute phase or at a later date. It usually manifests as acute or subacute meningitis. Pyogenic involvement of brain parenchyma causing abscess is an uncommon manifestation. We have diagnosed and treated a case of cerebellar epidermoid cyst secondarily infected with Brucella in a young healthy adult in our institute with combined surgical and medical intervention.
Keywords
Brucellosis
Infected epidermoid cyst
Cerebellum
Surgical drainage
Combination antibiotics
INTRODUCTION
Brucellosis, otherwise known as “undulant fever,” “Mediterranean fever,” or “Malta fever,” is the most common zoonotic infection. It is transmitted to humans from infected cattle, sheep, goats, camels, pigs, or other animals. The most common mode of entry is ingesting animal food products such as unpasteurized dairies. The less common mode is contact with infected tissues such as parturition products or body fluids such as blood, urine, and milk, or through inhalation.[1-3] Brucellosis is thus an occupational disease in shepherds, abattoir workers, veterinarians, dairy-industry professionals, and laboratory personnel. Rare cases of human-to-human transmission through blood transfusion, tissue transplantation, breastfeeding, sexual contact, congenital transmission, and nosocomial infection have been described.[4-7]
After the acute phase, untreated infection may manifest as one or more focal organ involvement. The likelihood of focal involvement ranges from (6-12)% and is usually about 30%.[8-10] Osteoarticular disease is the most common form of focal brucellosis; it occurs in up to 70% of patients.[11,12] Neurologic involvement may occur in up to 10% of cases.[13,14] Manifestations include acute or chronic meningitis, encephalitis, brain abscess, myelitis, radiculitis, and/or neuritis with involvement of cranial or peripheral nerves.[15-17] Relapse following treatment is seen in 5–15% of cases. It usually occurs within the first 6 months following completion of treatment but may occur up to 12 months post-treatment.[18]
CASE REPORT
A 24-year-old male, student of mass communication, presented with complaints of headaches for 3 weeks. It was insidious in onset, gradually progressive, and holocranial in nature. It was not associated with vomiting, blurring of vision, or diplopia. He had fever for the same duration which was low-grade, intermittent, and used to get subsided with medications; there was no history of loss of consciousness, altered sensorium, seizures, or posturing-like episodes. There was no history of pain in the spine or peripheral joints. He had no known comorbid illness, and had no history of significant animal contact or ingestion of unpasteurized milk. On examination, pulse rate and blood pressure were 80/min and 100/80 mmHg, respectively. The general and systemic examinations were normal. There was a bilateral horizontal gaze-evoked nystagmus with coarse on looking to the left. There was no lateral rectus palsy. Cranial nerves were normal. He had signs of cerebellar dysfunction on the right. There was no evidence of meningeal irritation. Baseline investigations revealed hemoglobin of 12.5 gm/dL, total leukocyte count of 8000/cu mm, and platelet count of 2.77 lacs/cu mm. Creatinine was 0.89 mg/dL, liver function test, and serum electrolytes were normal. C-reactive protein (CRP) was not elevated. The blood culture was sterile. Blood-borne virus screen was negative. Computed tomography of brain showed a right cerebellar hemispheric cystic mass with internal calcification with mild upstream hydrocephalus [Figure 1a and b]. Post-contrast magnetic resonance imaging (MRI) brain revealed a well-marginated cystic mass in the right cerebellar hemisphere involving the middle cerebellar peduncle [Figure 1c]. T2-weighted MR demonstrates the cystic nature of the lesion with involvement of the right middle cerebellar peduncle and an exophytic component along the right side of the pons [Figure 1d and e]. The diffusion sequence showed internal diffusion restriction with marginal post-contrast enhancement [Figure 1f]. There was mild surrounding edema. Given the cystic nature of the mass and internal diffusion restriction, an epidermoid cyst was considered; however, the presence of calcification and wall enhancement were atypical. He underwent right retromastoid suboccipital craniotomy and near-total excision of the mass lesion. Intraoperatively, the bone was thinned out. The dura was tense and bulging. There was an encapsulated pus-filled cavity in the right cerebellar hemisphere at a depth of around 2 cm. After evacuating the pus, layers of flaky, pearly white materials suggestive of the epidermoid cyst were seen. Materials were sent for histopathology and cultures. He did well post-operatively and was shifted to the ward on day 3. Xpert Mycobacterium tuberculosis/rifampicin, fungal stain, and culture were negative. Gram stain showed minimal pus cells and a few Gram-negative rods. Histopathology of the excised tissues showed lamellated keratinized materials lined by stratified squamous epithellium. Cyst wall was infiltrated with neutrophils, macrophages, and lymphocytes.
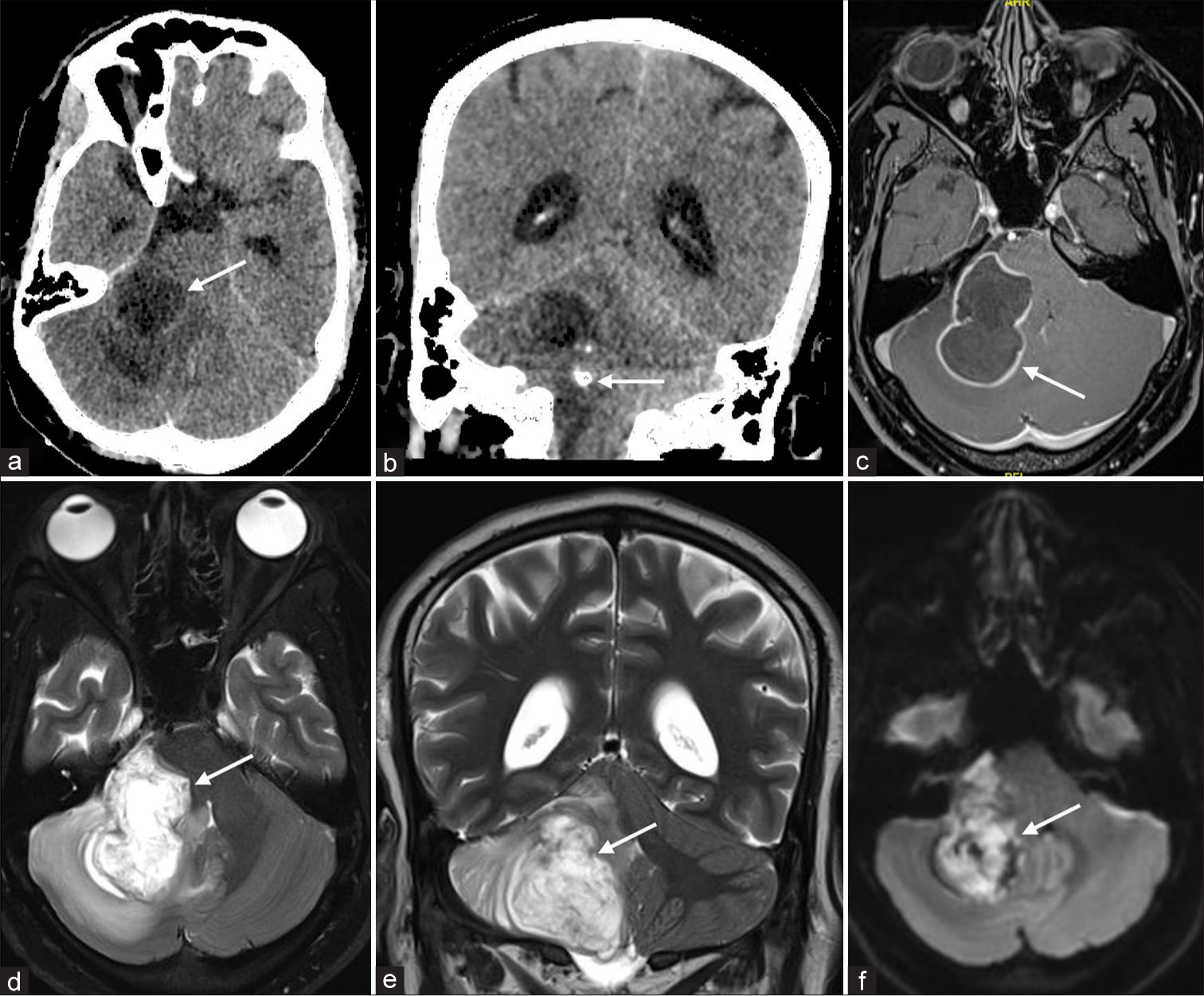
- (a) Computed tomography (CT) shows a cystic mass like lesion in the right cerebellar hemisphere (white arrow). (b) CT demonstrates an eccentric nodular calcification (white arrow). T1W post contrast MR, (c) shows peripheral wall enhancement (white arrow). T2 weighted axial and coronal MR images (d and e respectively) demonstrates cystic nature of the lesion with involvement of the right middle cerebellar peduncle and an exophytic component along the right side of pons (white arrows). Diffusion sequence (f) shows central restriction diffusion (white arrow). MR: Magnetic resonance.
He was initiated and continued on injection cefoperazonesulbactam postoperatively. On day 13, the bacterial culture grew a Gram-negative organism later confirmed as Brucella abortus. Antibiotic was switched to injection gentamicin and oral doxycycline, and was referred to the infectious disease clinic. We reviewed him and Brucella serology was sent which came negative. Multiple blood cultures did not isolate any pathogen. Microbiology was asked for the antimicrobial susceptibilities [Table 1].
| Antibiotics | MIC | Interpretation |
|---|---|---|
| Co-trimoxazole | 2 μg/mL | Susceptible |
| Gentamicin | 2 μg/mL | Susceptible |
| Doxycycline | 0.12 μg/mL | Susceptible |
MIC: Minimum inhibitory concentration.
Antibiotics were changed to an injection of ceftriaxone 2 g intravenous twice a day, tablet doxycycline 100 mg twice a day, and capsule rifampicin 600 mg once a day according to the largest retrospective study reported by Erdem et al., from Istanbul.[19] He did well with the combination. The fever subsided completely, headache resolved and nystagmus improved. Ceftriaxone was stopped after 4 weeks and oral doxycycline with rifampicin was continued for a total of 12 weeks. He was kept under follow-up and is currently doing well after 3 months of treatment completion.
DISCUSSION
The central nervous system (CNS) is involved in 4 – 11% of cases in the course of brucellosis. It was first reported by Hughes in 1896. The manifestations of neurobrucellosis are quite heterogeneous. Buzgan et al. reported 5.6% focal brain involvement in their retrospective review of 1028 cases.[20] Of these, seven had encephalitis (12.1%), two had myelitis (3.4%), two had polyradiculoneuritis (3.4%), one had a hypophysis abscess (1.7%), and one had a frontal abscess (1.7%). The remaining 45 patients had either meningitis or meningoencephalitis (77.6%). Gul et al. in their review of 187 cases in Turkish medical practices reported only three cases of brain abscesses whereas meningitis was most common manifestation.[21]
Isolation of organisms in neurobrucellosis is often not possible. The majority are diagnosed by compatible clinical syndrome, radiology, and CSF serology. In our case, the organism grew from the purulent materials recovered intraoperatively. Pre-operative serology was not performed as his clinical syndrome and radiology pointed toward a cerebellopontine angle neoplasm or cystic mass. Serology was performed 3 weeks after the source was controlled. There was no clinical or radiological evidence of meningitis. These two may be the reason for his negative Brucella serology. He was non-bacteremic and did not have any other focal organ involvement. His clinical presentation was not compatible with an abscess as he had minimal fever, normal CRP, and a prominent headache due to mass effect. Diffusion sequences on MRI showed a variegated appearance of the lesion with minimal rim enhancement and unimpressive surrounding edema, unlike classical brain abscess. As reported above, brain abscess is an uncommon presentation of neurobrucellosis and the majority present with meningitis or meningoencephalitis. In our case, there was a cerebellopontine angle epidermoid cyst that got infected secondarily resulting in an abscess-like lesion. It might have occurred in the recent past during bacteremia. A zoonotic organism infecting a pre-existing brain cyst made it unique and interesting.
Treatment of neurobrucellosis is based upon observational studies. The majority were treated with a combination of ceftriaxone, rifampicin, and doxycycline for initial 4–6 weeks. Once improved, the oral rifampicin and doxycycline need to be continued for 3–6 months.[19,22] In our case, the isolate was pansusceptible to cotrimoxazole, gentamicin, and doxycycline [Table 1] used to treat brucellosis. Ceftriaxone does not have a minimum inhibitory concentration (MIC) interpretation for brucellosis. He received ceftriaxone for the initial 4 weeks of intensive phase alongwith doxycycline and rifampicin. The latter two were continued for another 8 weeks in continuation phase. He did well with this combination and is currently under follow-up.
CONCLUSION
Intracranial epidermoid cysts may sometimes mimic brain abscess after getting secondarily infected by the microorganisms. Radiologically it is not easy to differentiate infected cyst from true abscess. Acute brucellosis may involve epidermoid cyst during bacteremic phase. It should be kept in etiological consideration in proper epidemiological settings.
TEACHING POINTS
Brucellosis should be considered by the treating physician whenever a space occupying lesion is encountered in brain.
It may form a frank abscess or can involve any preexisting lesion in brain parenchyma.
Radiological differentiation is not always easy and obtaining tissue or pus is crucial to make a definitive diagnosis.
MCQs
-
Which is the most common manifestation of nervous system involvement by brucella?
Myelitis
Meningoencephalitis
Radiculitis
Brain abscess
Answer key – b
-
Rifampicin is NOT used in which of the following infections?
Mycobacterium tuberculosis
Brucellosis
Staphylococcal aureus
Streptococcus pyogenes
Answer key – d
-
Which injectable antibiotic works best in neuro brucellosis?
Gentamicin
Amikacin
Ceftriaxone
Ceftazidime
Answer key – c
Acknowledgement
Department of Neurosurgery, Department of Pathology and Department of Microbiology.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- WHO/CDS/EPR/2006.7 In: Brucellosis in humans and animals. Geneva: World Health Organization; 2006.
- [Google Scholar]
- Brucellosis reference guide. 2017. United States: CDC; Available from: https://www.cdc.gov/brucellosis/pdf/brucellosi-reference-guide.pdf [Last accessed on 2024 Mar 27]
- [Google Scholar]
- A rare case of Brucella melitensis infection in an obstetrician during the delivery of a transplacentally infected infant. J Infect. 2006;53:e39-41.
- [CrossRef] [PubMed] [Google Scholar]
- The many faces of human-to-human transmission of brucellosis: Congenital infection and outbreak of nosocomial disease related to an unrecognized clinical case. Clin Infect Dis. 2007;45:e135-40.
- [CrossRef] [PubMed] [Google Scholar]
- Brucella melitensis--a sexually transmissible agent? Lancet. 1996;347:1763.
- [CrossRef] [PubMed] [Google Scholar]
- Potential risk of blood transfusion-transmitted brucellosis in an endemic area of China. Transfusion. 2015;55:586-92.
- [CrossRef] [PubMed] [Google Scholar]
- Human brucellosis in Macedonia-10 years of clinical experience in endemic region. Croat Med J. 2010;51:327-36.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features of 2041 human brucellosis cases in China. PLoS One. 2018;13:e0205500. Erratum in: PLoS One 2019;14:e0211102, Erratum in: PLoS One 2019;14:e0213558. Erratum in: PLoS One 2019;14:e0219110
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological features and clinical manifestations in 469 adult patients with brucellosis in Babol, Northern Iran. Epidemiol Infect. 2004;132:1109-14.
- [CrossRef] [PubMed] [Google Scholar]
- Osteoarticular involvement in brucellosis: Study of 196 cases in the Republic of Macedonia. Croat Med J. 2004;45:727-33.
- [Google Scholar]
- Musculoskeletal involvement of brucellosis in different age groups: A study of 195 cases. Swiss Med Wkly. 2002;132:98-105.
- [CrossRef] [PubMed] [Google Scholar]
- Update on laboratory diagnosis of human brucellosis. Int J Antimicrob Agents. 2010;36(Suppl 1):S12-7.
- [CrossRef] [PubMed] [Google Scholar]
- Complications and treatment of brucellosis: 11-year results. Acta Med Mediterr. 2019;35:1131.
- [Google Scholar]
- Review of clinical and laboratory features of human brucellosis. Indian J Med Microbiol. 2007;25:188-202.
- [CrossRef] [PubMed] [Google Scholar]
- Brucellosis in 418 patients from the Balkan Peninsula: Exposure-related differences in clinical manifestations, laboratory test results, and therapy outcome. Int J Infect Dis. 2007;11:342-7.
- [CrossRef] [PubMed] [Google Scholar]
- Perspectives for the treatment of brucellosis in the 21st century: The Ioannina recommendations. PLoS Med. 2007;4:e317.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and tolerability of antibiotic combinations in neurobrucellosis: Results of the Istanbul study. Antimicrob Agents Chemother. 2012;56:1523-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical manifestations and complications in 1028 cases of brucellosis: A retrospective evaluation and review of the literature. Int J Infect Dis. 2010;14:e469-78.
- [CrossRef] [PubMed] [Google Scholar]
- Overview of neurobrucellosis: A pooled analysis of 187 cases. Int J Infect Dis. 2009;13:e339-43.
- [CrossRef] [PubMed] [Google Scholar]
- Central nervous system brucellosis: Presentation, diagnosis and treatment. J Infect. 1998;36:297-301.
- [CrossRef] [PubMed] [Google Scholar]







