Translate this page into:
From pancreas to pleura: Unraveling a rare complication
*Corresponding author: M. Archana, Department of Radiodiagnosis, Shridevi Institute of Medical Sciences and Research Hospital, Tumkuru, Karnataka, India. docarchanam@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Archana M, Divya J, Amruth VC, Manju MD. From pancreas to pleura: Unraveling a rare complication. Case Rep Clin Radiol. doi: 10.25259/CRCR_69_2024
Abstract
Pancreaticopleural fistulas (PPFs) are a rare and challenging condition in both clinical and diagnostic aspects. It is a rare complication of pancreatitis resulting from pancreatic collections communicating with the pleural cavity. They primarily cause respiratory system symptoms and pleural effusion. We present a case of a 30-year-old male with alcohol-induced pancreatitis presented with 2 months history of shortness of breath and cough. Chest radiograph demonstrated right massive pleural effusion. Repeated thoracentesis showed recurrent hemorrhagic pleural fluid with increased amylase and lipase. Computed tomography revealed no significant cause, and magnetic resonance cholangiopancreatography showed a fistulous tract between the pancreas and the right pleural cavity. The patient’s clinical presentation was misleading due to respiratory complaints, and a high clinical suspicion of PPF in recurrent massive hemorrhagic pleural effusion will lead to this diagnosis.
Keywords
Pancreaticopleural fistula
pancreatitis
Magnetic resonance cholangiopancreatography
Hemorrhagic pleural effusion
INTRODUCTION
Pancreaticopleural fistula (PPF) is a rare complication of chronic pancreatitis, often resulting in recurring pleural effusions due to abnormal communication from the pancreatic duct or pseudocyst. Recognizing characteristic clinical and imaging findings is crucial for accurate and early diagnosis. Minimally invasive endoscopic intervention is typically the first choice before invasive surgical management.[1]
The presence of a PPF is not typically associated with active acute pancreatitis. Patients with fistulae often have a history of chronic relapsing pancreatic inflammation. Other causes include gallstones, trauma, or pancreatic duct anomalies. The fistula can be detected through magnetic resonance cholangiopancreatography (MRCP) or computed tomography (CT) imaging, but the fistula connecting the pleural cavity and the abdominal cavity is difficult to visualize. The biochemical pleural fluid analysis may provide more definitive results, revealing a significant increase in amylase and lipase activity. PPF, due to its low incidence, is often overlooked as a cause of pleural effusion, making it a challenging diagnosis both clinically and radiologically.[2]
This case was studied in Shridevi Institute of Medical Sciences and Research Hospital, Tumkur, Karnataka, India. The patient presented to the Department of Respiratory Medicine with respiratory complaints. Routine investigations, chest radiography, diagnostic pleural fluid analysis, and therapeutic thoracocentesis were done. The patient was referred to the Department of radiodiagnosis for further investigations. CT was performed on 16-slice multidetector CT (WIPRO GE HEALTHCARE), plain and contrast CT (IV non-ionic contrast 350 mg of iodine at the rate of 3 mL/s at a delay of 40 s), and MRCP was performed on GE healthcare Signa HDxT 3.0 T magnetic resonance imaging (MRI).
CASE REPORT
A 30-year-old male, a chronic alcoholic and smoker, was admitted to the hospital with a dry cough, chest pain, shortness of breath, weight loss, and appetite loss for 2 months. Examination revealed a dull note on percussion and reduced air entry on the right side, suggesting right-sided pleural effusion, while clinical examination of the abdomen, cardiovascular system, and central nervous system was normal.
The routine investigation reports were as follows:
Hemoglobin – 8.8 g/dL, Total leukocyte count (TC) – 4400 cells/mm3 with lymphocytosis, serum amylase – 422 U/L, and serum lipase – 380 IU/L.
The initial chest radiograph posteroanterior view showed a right-sided massive pleural effusion. The patient underwent thoracentesis, removing approximately 1 L of brown fluid from the right pleural space. Diagnostic pleural fluid analysis revealed that hemorrhagic pleural fluid was with protein – 3.1 g/dL and sugar – 84 mg/dL; lactate dehydrogenase – 310 U/L, adenosine deaminase – 18 U/L; and no malignant cells. The pleural fluid amylase and lipase were enormously high, with 8717 U/L and 1259 IU/L, respectively [Figure 1].
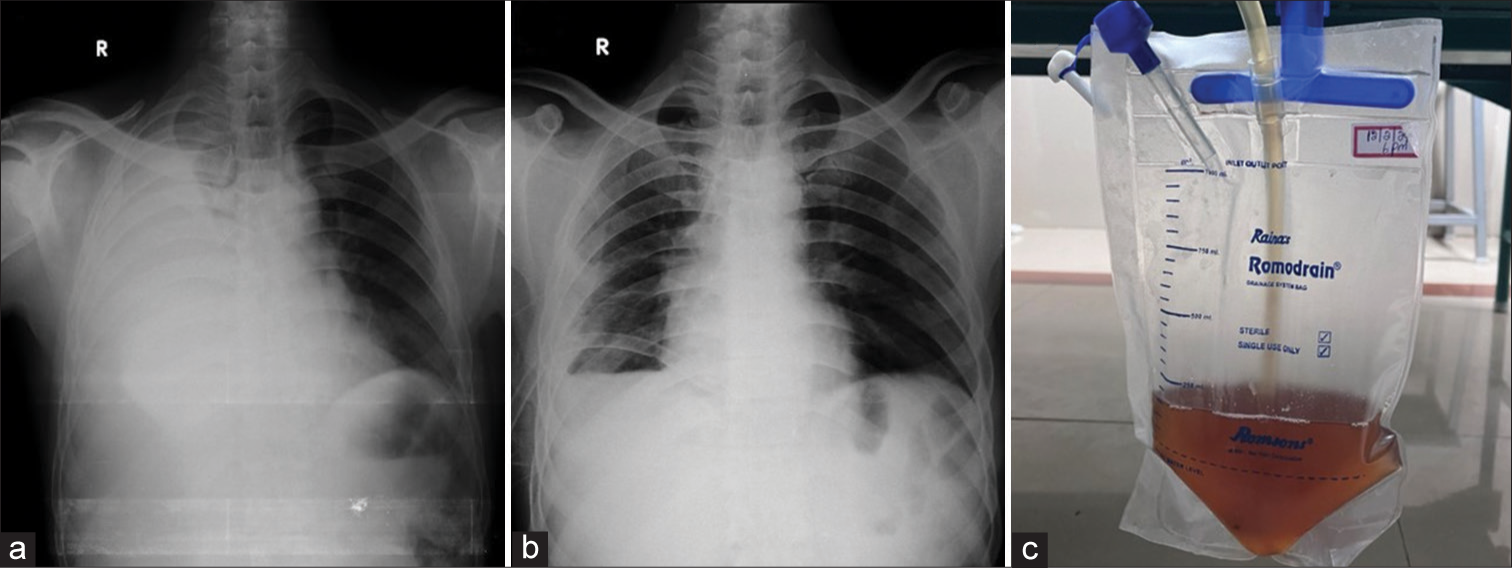
- (a) Chest radiograph at the time of admission showing massive right pleural effusion, (b) chest radiograph after thoracocentesis showing intercostal drainage tube in situ and reduction in pleural effusion, and (c) hemorrhagic pleural fluid.
Contrast-enhanced CT of the thorax and upper abdomen revealed gross right-sided pleural effusion causing underlying lung collapse. The visualized abdomen showed two non-enhancing hypodense areas in the uncinate process and body of the pancreas, the largest measuring ~15 mm in size. The main pancreatic duct was normal – likely representing pancreatic pseudocysts [Figure 2].
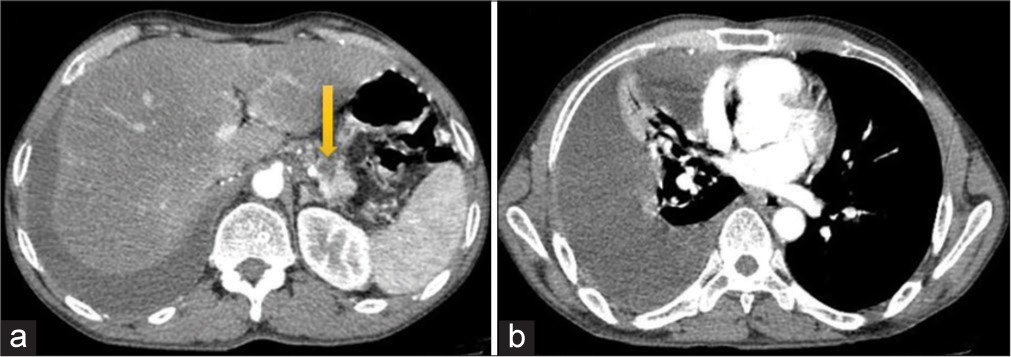
- (a) Contrast-enhanced computed tomography showing pseudocyst (yellow arrow) in the body of the pancreas. (b) Gross right pleural effusion.
MRI of the abdomen and MRCP revealed a fistulous tract communicating between the pancreatic pseudocyst of the body (defect size measuring 3.4 mm on the superior aspect) of the pancreas and the right pleural cavity (lower mediastinal aspect). The length of the fistulous tract measured ~10 cm and with a maximum diameter of ~10 mm. The tract was seen passing through the esophageal hiatus to enter the right pleural cavity with significant adjacent peri-fistulous inflammatory changes at D9–D11 prevertebral regions [Figure 3].
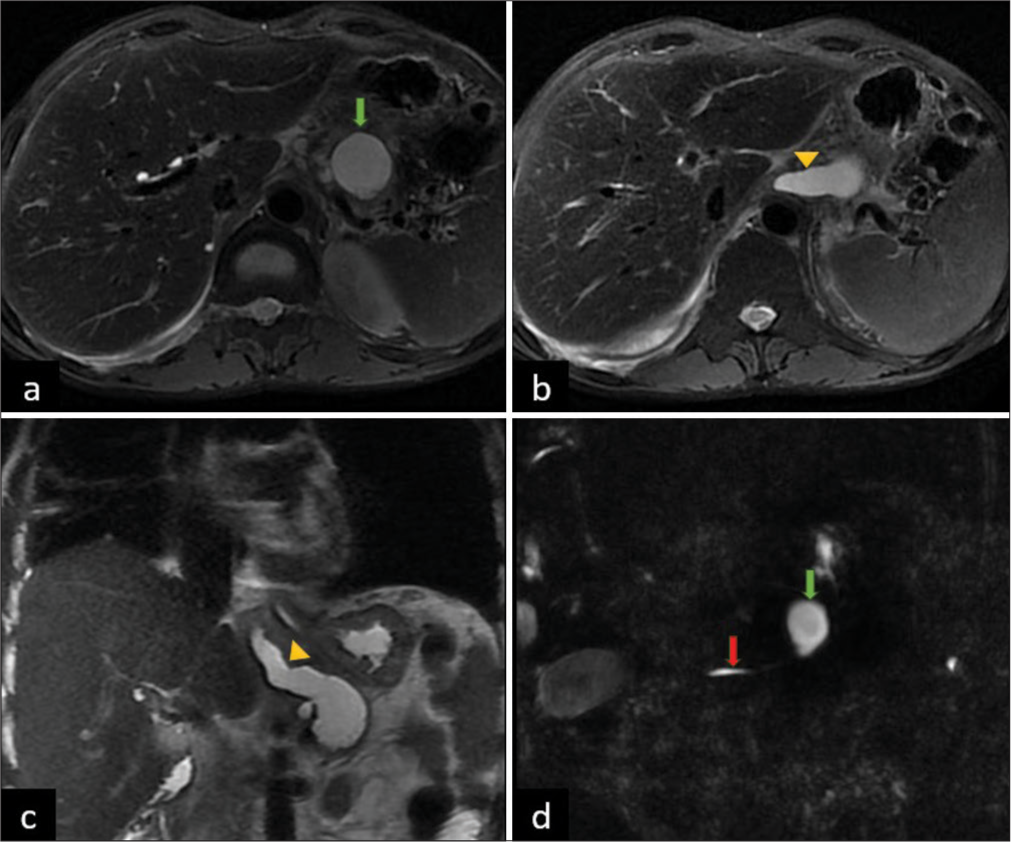
- (a) Axial T2 MRI showing pseudocyst in pancreatic body (green arrow). (b) Linear non ramifying fistulous tract (yellow arrowhead). (c) Coronal T2 MRI showing fistula (yellow arrowhead) extending into right pleural cavity. (d) Magnetic resonance cholangiopancreatography (MRCP) image showing communication of pseudocyt (green arrow) with main pancreatic duct (red arrow).
The patient was subsequently referred to the Department of Surgical Gastroenterology for further evaluation and management. A surgical resection of the fistulous tract was performed successfully, and the patient was discharged without any further complications.
DISCUSSION
PPF, a rare complication of chronic pancreatitis, presents a diagnostic challenge due to patients’ primarily thoracic symptoms, often causing delays in diagnosis. Pancreatic fistulas tend to occur in 0.4% of patients with chronic pancreatitis and 4.5% in patients with pseudocysts.[3]
Although a high pleural fluid amylase is attributable to pleural effusions related to chronic pancreatitis, it can also be due to esophageal perforation, acute pancreatitis, parapneumonic effusions, and pancreatic malignancy. However, pleural fluid amylase levels >5000 IU/L are strongly suggestive of PPF.[4] Pleural effusion can occur due to two mechanisms in the setting of pancreatic pathologies. The first type (reactionary) is usually small, seen in the left pleural cavity with normal amylase activity (below 100 U/L) and low protein content (below 3 g/dL). This type is associated with acute pancreatitis and is resolved during recovery.[5,6] The second type is the presence of PPF in relation to chronic or recurrent pancreatitis, which has the potential to invade the pleura, bronchi, mediastinum, or pericardium due to the enzymatic properties of the pancreatic fluid and the gradients of pressure between the abdomen and the thorax. This effusion is usually massive, unilateral, and recurrent and has a high level of pleural fluid amylase (usually over 1000 U/L) and proteins (over 3 g/dL).[7]
The basic mechanism that causes PPF formation is typically a direct pancreatic duct leak or a leak from a burst or incomplete pseudocyst. An anterior pancreatic duct disruption that is not walled off may result in the development of a pancreaticperitoneal fistula and presents as ascites. Conversely, a posterior disruption will pass through the retroperitoneum and can either appear as a mediastinal pseudocyst, which then bursts into the pleural cavity and becomes a PPF, or it can dissect through the aorta or esophageal hiatus into the mediastinum resulting in a pleural fistula.[8]
The initial presentation of PPF is typically middle-aged men with a history of alcohol abuse and increased levels of amylase and protein in their pleural fluid. However, most cases are not linked to acute pancreatitis episodes despite the high serum activity of amylase in most patients. This may be due to amylase being reabsorbed from the pleural space.[5]
The diagnosis of PPF generally relies on clinical clues and imaging. A CT scan is frequently the first pancreatic imaging investigation performed after the initial chest X-ray and thoracentesis in these patients. Tombroff et al.[9] injected a contrast agent into the pleural cavity in three patients and monitored the contrast material’s progress with fluoroscopy. A fistulous tract was demonstrated in two out of three patients. The article emphasizes the diagnostic significance of injecting contrast medium early into the pleural cavity in cases of amylase-rich effusion before lung expansion obscures the fistula. A transabdominal ultrasound can be used to make the initial diagnosis, which can then be confirmed by CT and endoscopic retrograde cholangiopancreatography (ERCP).[3]
When PPF is suspected, MRCP is considered to be the investigation of choice. Non-invasiveness and the ability to identify a fistula, even in severe pancreatic duct strictures, are two benefits of MRCP. It permits mapping of the ductal anatomy in conjunction with pathological changes in adjacent structures, which can provide crucial information for appreciating local anatomy and implementing the best interventional plan. MRCP’s sensitivity has been estimated to be 80%. Several pseudocysts or ascites can result in false negative results by lowering image quality.[2] The basis of MRCP is the acquisition of T2-weighted images, which can demonstrate the entire fistulous tract as a structure with a high signal intensity. MRCP is less invasive than ERCP and typically does not require sedation or contrast administration. The link between the fistulous tract and the pancreatic duct can now be distinguished more clearly due to recent advancements and better-resolution magnetic resonance imaging techniques.[4]
ERCP is the second most effective modality to diagnose PPF, with a sensitivity of 46–78%. It serves as both a diagnostic and therapeutic tool for pancreatic and biliary diseases. However, significant ductal strictures or obstructions and anatomic variations of the pancreatic ducts may render this approach ineffective.[2] It is an operator-dependent technique associated with an increased risk of procedure-related pancreatitis. If there is a pancreatic duct obstruction, it can potentially miss a PPF.[3]
Surgery, endoscopic management, and conservative treatment are available therapeutic options for PPF. Somatostatin analogs are typically used for 2–3 weeks of medical treatment to lower pancreatic production and pressure in the pancreatic ducts. This approach is estimated to have a 30–60% success rate.[10] ERCP enables simultaneous fistula assessment and duct decompression through endoscopic procedures such as sphincterotomy, stone removal, balloon stricture dilatation, and stenting.
Surgery is required for patients who have a failure of non-invasive treatment. These patients generally have severe pancreatic duct strictures and multiple polymorphic pseudocysts. Depending on the anatomy and location of involvement, distal pancreatectomy or pancreatojejunostomy of fistula closure through a transthoracic approach is carried out.
CONCLUSION
PPF should be suspected in patients presenting with recurrent massive hemorrhagic pleural effusion, particularly those with a history of pancreatitis or pancreatic surgery. Elevated pleural fluid amylase levels remain the primary laboratory diagnostic method for identifying this condition. While CT is the first line of cross-sectional imaging for evaluating pleural effusion, MRCP is considered the investigation of choice for suspected PPF due to its high sensitivity, which is approximately 80%.
TEACHING POINTS
Clinical Suspicion: PPF should be suspected in patients presenting with recurrent massive hemorrhagic pleural effusion, especially if there is a history of pancreatitis or pancreatic surgery.
Laboratory Diagnosis: Elevated pleural fluid amylase levels remain the primary laboratory diagnostic method for identifying PPF.
Imaging Evaluation: While CT is the first line of cross-sectional imaging for evaluating pleural effusion, MRCP is considered the investigation of choice for suspected PPF due to its high sensitivity, approximately 80%.
MCQs
-
What is the primary laboratory diagnostic method for identifying PPF?
Elevated serum lipase levels
Elevated pleural fluid amylase levels
Elevated serum amylase levels
Elevated pleural fluid protein levels
Answer Key: b
-
Which imaging modality is considered the investigation of choice for suspected PPF due to its high sensitivity?
CT
ERCP
MRCP
Transabdominal Ultrasound
Answer Key: c
-
Which of the following is NOT a common cause of elevated pleural fluid amylase levels?
Esophageal perforation
Parapneumonic effusions
Acute pancreatitis
Hepatic cirrhosis
Answer Key: d
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Pancreaticopleural fistula: A review of imaging diagnosis and early endoscopic intervention. Case Rep Gastrointest Med. 2018;2018:7589451.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreaticopleural fistulas of different origin: Report of two cases and a review of the literature. Pol J Radiol. 2011;76:56-60.
- [Google Scholar]
- Thoracopancreatic fistula: Clinical and imaging findings. J Comput Assist Tomogr. 1999;23:181-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreatic-pleural fistula is best managed by early operative intervention. Surgery. 2010;147:154-9.
- [CrossRef] [PubMed] [Google Scholar]
- Routine measurement of pleural fluid amylase is not indicated. Arch Intern Med. 2001;161:228-32.
- [CrossRef] [PubMed] [Google Scholar]
- Pancreaticopleural fistula and mediastinal pseudocyst: An unusual presentation of acute pancreatitis. Ann Thorac Med. 2007;2:122-3.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management and treatment of pancreatic fistulas. Surg Clin North Am. 1996;76:1159-73.
- [CrossRef] [PubMed] [Google Scholar]
- Pleural effusion with pancreaticopleural fistula. Br Med J. 1973;1:330-1.
- [CrossRef] [PubMed] [Google Scholar]
- Internal pancreatic fistulas: Proposal of a management algorithm based on a case series analysis. J Clin Gastroenterol. 2004;38:795-800.
- [CrossRef] [PubMed] [Google Scholar]







