Translate this page into:
Complex fetal cardiac anomalies: Transposition, anomalous venous connection, and atresias
*Corresponding author: Praveenkumar Rathinamoorthy, Department of Radiology and Imaging Sciences, Bhaarath Medical College and Hospital, Chennai, Tamil Nadu, India. pr23vikmr@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rathinamoorthy P, Farook A. Complex fetal cardiac anomalies: Transposition, anomalous venous connection, and atresias. Case Rep Clin Radiol. doi: 10.25259/CRCR_180_2023
Abstract
This case report details the sonographic diagnosis of fetal complete transposition of the great arteries with anomalous pulmonary venous connection, mitral atresia, and pulmonary atresia during a 22-week gestation. Detailed fetal echocardiography revealed distinct cardiac anomalies characterized by abnormal spatial positioning of the great arteries, increased distance between the left atrium and the descending aorta, and completely underdeveloped left heart chambers. Precise identification of cardiac structures through detailed sonographic imaging facilitated the confirmation of the diagnosis. This report underscores the clinical value of detailed imaging in accurately detecting complex fetal cardiac abnormalities for improved prenatal counseling and informed decision-making.
Keywords
Complete transposition of great arteries
Mitral atresia
Ultrasound fetal echo
anomalous pulmonary venous connection
Pulmonary atresia
INTRODUCTION
Congenital heart defects (CHDs) represent a spectrum of anatomical anomalies affecting the structure and function of the heart. Among these, complete transposition of the great arteries (TGAs) accounts for 5–7% of all CHD and is characterized by a discordant connection between the ventricles and their respective arterial trunks.[1] When coupled with additional anomalies such as anomalous pulmonary venous connection (APVC), mitral atresia, and pulmonary atresia, the clinical complexity escalates, posing significant challenges in both prenatal diagnosis and management.
Complete TGA is characterized by a complete reversal of the aorta and pulmonary artery, leading to two separate parallel circulations, while APVC involves abnormal connections between the pulmonary veins and the heart. The oxygen-rich blood is ultimately channeled to the aorta, which leads to less severe cyanosis. However, this condition is again complicated by the presence of mitral and pulmonary atresia with severe underdevelopment of the left atria and left ventricle (LV) of the heart. This case report describes a prenatal diagnosis of both conditions in combination and highlights the challenges and considerations in managing such complex CHDs.
CASE REPORT
A 28-year-old primigravida presented at 22 weeks of gestation for a routine prenatal anomaly assessment. The patient came for an anomaly assessment without any prior scans since confirming pregnancy. Her past and family histories were unremarkable. Her marriage was non-consanguineous.
Imaging finding
A transabdominal sonogram of the fetal heart revealed normal situs with a moderately dilated right atrium and right ventricle (RV) alongside severe underdevelopment of the left atrium and LV in apical four-chamber view. The morphology of the RV is confirmed by the presence of a moderator band and the septal attachment of the leaflets. The four- chamber view of the hear also demonstrated an increased interval between the left atrium and the descending aorta with a linear abnormal single vessel in between them suggesting APVC [Figure 1a].
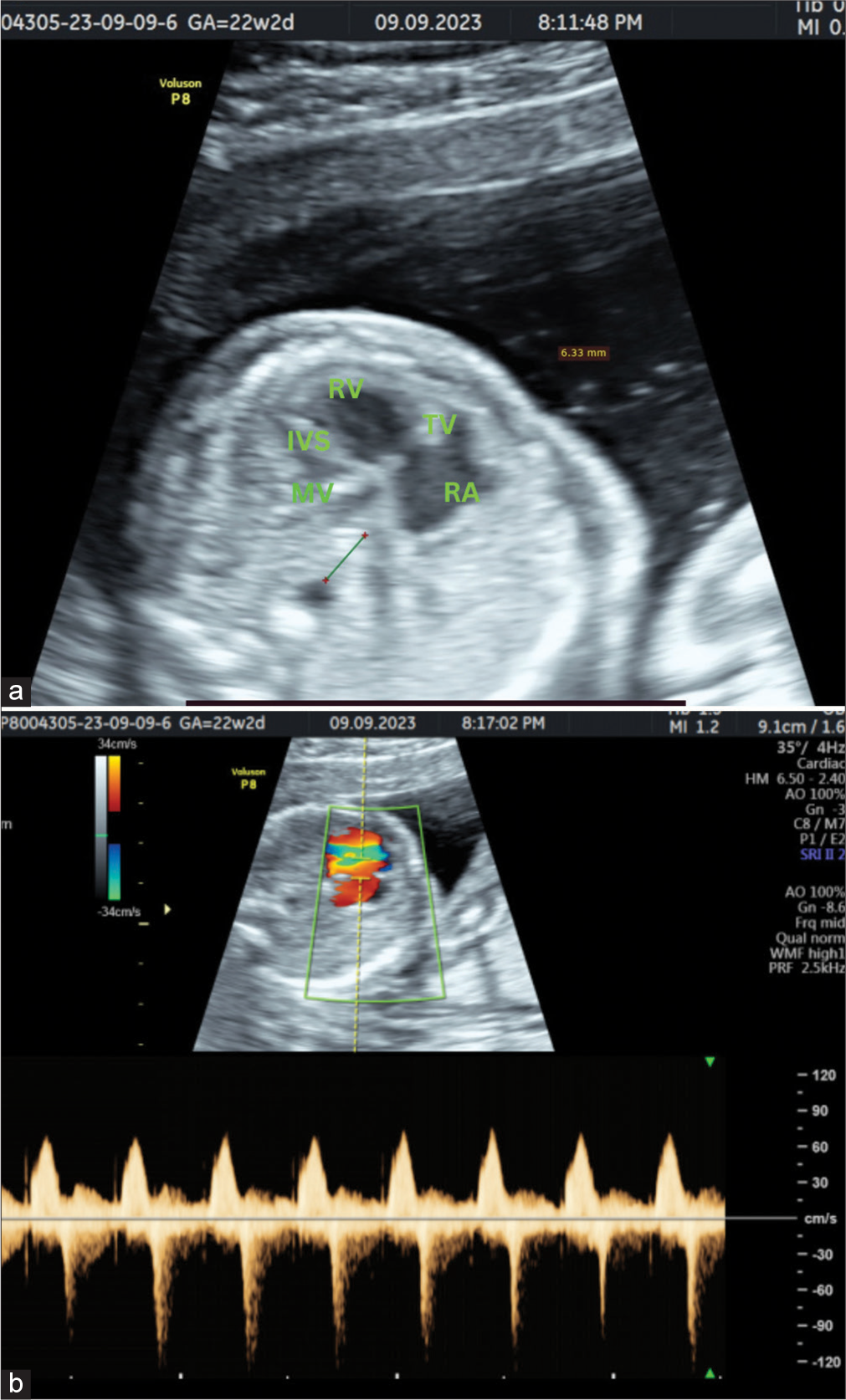
- A 20-year-old female patient came for anomaly assessment. (a) Four-chamber view shows dilated right ventricle and right atrium with severely hypoplastic left heart chambers. There is also increased distance between descending aorta and the left atrium with abnormal vessel in between. (b) Spectral Doppler image of four-chamber view shows mild tricuspid regurgitation. RA: Right atrium; RV: Right ventricle; IVS: Interventricular septum; MV: Mitral valve; TV: Tricuspid valve.
There is also a dysplastic tricuspid valve with mild tricuspid regurgitation [Figure 1b].
The outlet tract views show a large arterial tract resembling an aorta seen arising from the dilated RV, with branches extending to the cranium, confirming complete TGA [Figure 2].
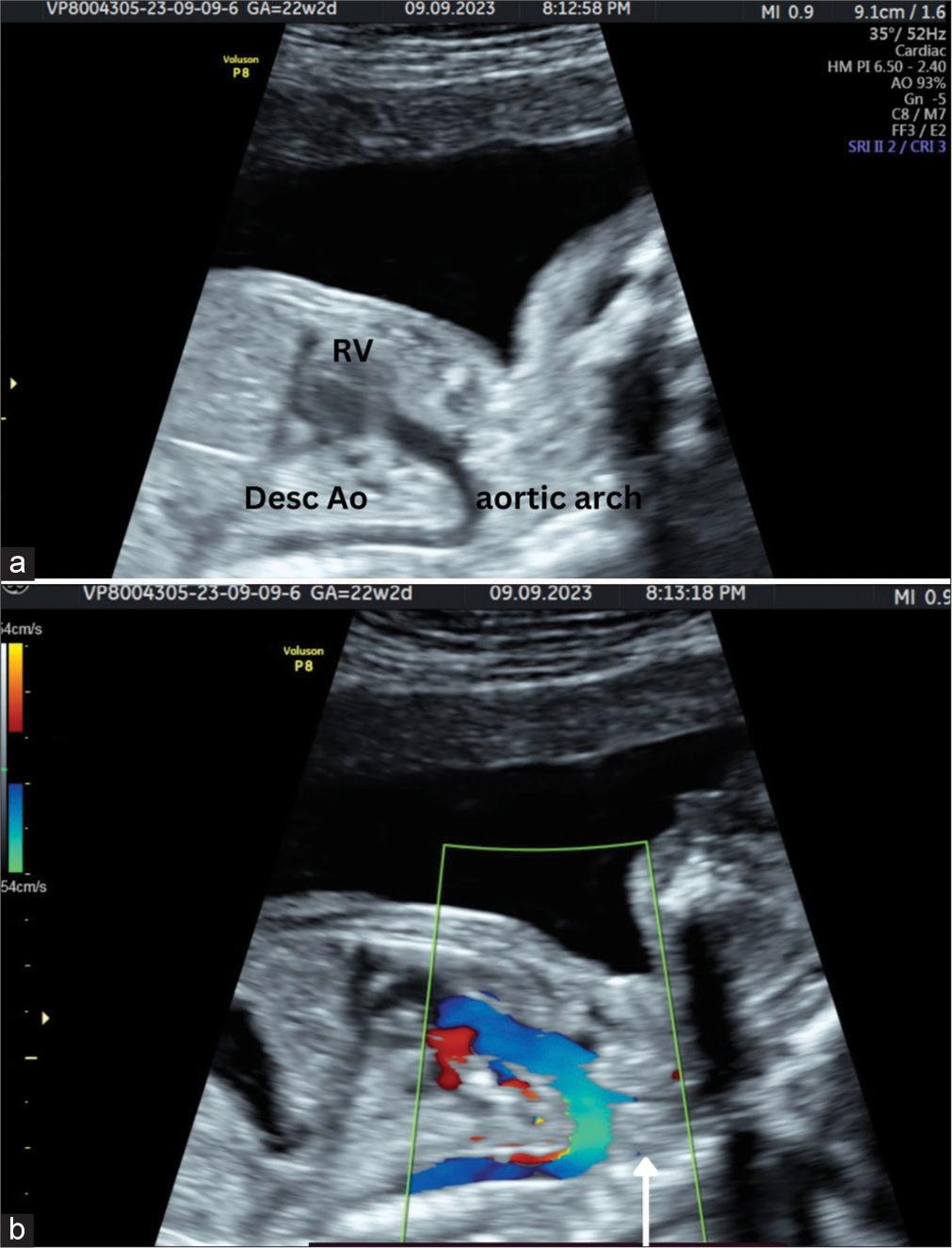
- A 20-year-old female patient came for anomaly assessment. (a) Gray scale image and (b) Spectral Doppler image of paramedial sagittal plane shows dilated aorta arising from right ventricle. The left common carotid artery is seen arising from aortic arch (white arrow). RV: Right ventricle.
The LV and the left atrium appear severely underdeveloped; hence, the possibility of mitral atresia and pulmonary atresia was suggested.
Due to the severely underdeveloped left atrium, differentiation into partial and complete anomalous venous connection was not possible. Thus, a final diagnosis of complete (d) TGA with APVC and associated mitral and pulmonary atresia was suggested.
There was no evidence of other major structural abnormalities.
DISCUSSION
TGAs are a CHD where the pulmonary artery and the aorta are switched in position. This results in oxygen-poor blood circulating in the body and oxygen-rich blood circulating back to the lungs, leading to severe cyanosis.
There are two main types of TGA: dextro-TGAs (D-TGAs) and levo-TGAs (L-TGAs).
In D-TGAs, there is atrioventricular concordance and ventriculoarterial discordance; thus, the aorta arises from the RV, and the pulmonary artery arises from the LV. This results in two separate circulatory loops where deoxygenated blood circulates from the right side of the heart to the body and oxygenated blood circulates from the left side of the heart to the lungs. This creates a parallel circulation, leading to cyanosis and reduced oxygen delivery to the body.
In L-TGAs, there is atrioventricular discordance and ventriculoarterial discordance; thus, the aorta arises from the morphologic RV which is on the left side and communicating with the left atrium and vice versa. Despite the abnormal anatomical arrangement, the circulation pattern is relatively normal. However, individuals with L-TGA are still at risk of developing heart rhythm abnormalities and heart failure later in life.
In D-TGA, the aorta is on the right side and the pulmonary artery is on the left, while in L-TGA, the positions are reversed. The type of TGA affects the management and prognosis of the condition, with D-TGA typically requiring surgical correction soon after birth for improved outcomes.[1]
Antenatal diagnosis depends on the assessment of the morphology of the ventricles in four-chamber view. The morphology of the RV is assessed by the presence of trabeculations, moderator bands, the relative apical position of the tricuspid valve, and the septal attachment of the valve.[2]
APVC is a CHD where abnormal connections occur between one or more pulmonary veins and the heart. In a normal heart, all four pulmonary veins should drain into the left atrium. However, in APVC, this pattern is disrupted, causing oxygen-rich blood from the lungs to drain abnormally into right atrium of systemic veins, thus leading to impaired circulation and complications.
Types of APVC include total APVC (TAPVC) and partial APVC (PAPVC).
In TAPVC, all pulmonary veins fail to connect to the left atrium and instead connect to the right atrium or other systemic veins. This results in a complete mixing of oxygen-rich and oxygen-poor blood within the heart, leading to cyanosis and impaired oxygen delivery to the body.
In PAPVC, one or more pulmonary veins connect to the right atrium or other systemic veins, while the remaining pulmonary veins connect normally to the left atrium. This results in a partial mixing of oxygen-rich and oxygen-poor blood depending on the number and location of the abnormal connections.[3]
APVC is diagnosed in a four-chamber view by looking at the left atrium and descending aorta relation. Normally, the left atrium is just anterior to the descending with no intervening vascular structures between them. In APVC, an abnormal vessel can be seen passing between the left atrium and descending aorta leading to an increased distance between the left atrium and descending aorta. In some scenarios, the abnormal vessel may not be so apparent but the clue to increased left atrial – descending aorta distance more than 3–5 mm.[4]
TAPVC and PAPVC can be further classified into supracardiac, cardiac, infracardiac, and mixed based on the location of the anomalous connection.
In the supracardiac subtype (the most common type of TAPVC), pulmonary veins connect, most commonly to the left innominate vein, but can also connect to the superior vena cava (SVC) or azygous vein.
In the cardiac subtype, pulmonary veins drain directly into the coronary sinus or right atrium.
In the infracardiac subtype, pulmonary veins form a vertical vein and drain into the portal vein, hepatic vein, or inferior vena cava (IVC) below the diaphragm.
The most common form of PAPVC involves the left upper pulmonary vein draining into the left innominate vein, ultimately leading to drainage into the right atrium. Other variations of PAPVC include pulmonary veins draining into the SVC, coronary sinus, IVC, or azygos veins.
Scimitar syndrome is a specific variant of PAPVC where part or all of the right lung is drained by the right pulmonary veins that connect to the IVC. The affected lung segments often display hypoplasia or bronchial anomalies.[3]
The isolated TGA or APVC usually presents with severe cyanosis, but the co-existence of complete TGA and APVC presents with less severe cyanosis and is relatively rare as there is some degree of congenital correction.[5,6] However, in this patient, the condition is further complicated by the presence of mitral and pulmonary atresia with a severely underdeveloped left atrium and LV.
Tricuspid regurgitation is diagnosed in four-chamber view by placing the pulsed Doppler gate with a sample volume of 3 mm perpendicular across the tricuspid valve, ensuring the angle of insonation is <20°. Diagnosis is confirmed when the color Doppler imaging reveals flow in the opposite direction accompanied by aliasing, indicating high-velocity flow and the regurgitation jet velocity reaches at least 80 cm/s and extends for more than half of the systole.[7]
Counseling the parents about the prognosis and treatment options was challenging, as the coexistence of multiple cardiac conditions significantly limited surgical and medical interventions.
Management
Given the complexity of this case, a multidisciplinary team was assembled and comprehensive prenatal counseling session was conducted with the parents to discuss the limited surgical options.
The delivery plan included choosing a tertiary care center with expertise in managing complex congenital heart diseases, ensuring neonatal intensive care unit availability, and arranging for neonatal transport services. In this case, the patient chose termination of pregnancy and was done successfully in the 2nd trimester.
CONCLUSION
The prenatal diagnosis of complete TGAs with APVC, mitral atresia, and pulmonary atresia is exceptionally rare but mandates prompt recognition and multidisciplinary care. Early and comprehensive counseling of parents, as well as meticulous planning for delivery and post-natal care, are essential in optimizing the outcomes for these infants. This case underscores the continued significance of detailed fetal echocardiography to enhance our ability to detect and comprehend complex fetal cardiac anomalies.
TEACHING POINTS
Antenatal diagnosis of TGA which is a cyanotic heart disease is of prime importance. Assessing the ventricular morphology, atrioventricular, and ventriculoarterial relation is basic in the diagnosis of TGA and its types.
D-TGA shows atrioventricular concordance with ventriculoarterial discordance, and L-TGA shows atrioventricular discordance with ventriculoarterial discordance.
APVC is diagnosed by the increased distance between the left atrium and the descending thoracic aorta, presence of abnormal vessel between the left atrium and the descending aorta, or absence of pulmonary vein connection with the left atrium.
Tricuspid regurgitation is diagnosed by the presence of flow in the opposite direction with aliasing artifact, regurgitation velocity more than 80 cm/s, and extends for more than half of the systole.
MCQs
-
The most specific sign for the diagnosis of TAPVC is:
Complete absence of pulmonary vein connection with the left atrium
Increased distance between the left atrium and descending aorta
Presence of abnormal vessels between the left atrium and descending aorta
None of the above.
Answer Key: a
-
Which among the following is the feature of tricuspid regurgitation?
Presence of an aliasing artifact in the opposite direction along the tricuspid valve
Peak systolic velocity more than 80cm/s
The regurgitant jet extends more than half of the systole
All of the above.
Answer Key: d
-
Which is the most common type of TAPVC?
Scimitar syndrome
Infracardiac
Cardia
Supracardiac
Answer Key: d
Acknowledgments
We would like to acknowledge the dedicated health-care professionals involved in the care of the patient and her family throughout this challenging journey.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Prenatal diagnosis of transposition of the great arteries: An updated review. Ultrasonography. 2020;39:331-9.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy of the normal fetal heart: The basis for understanding fetal echocardiography. Ann Pediatr Cardiol. 2018;11:164-73.
- [CrossRef] [PubMed] [Google Scholar]
- Partial and total anomalous pulmonary venous connection In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/books/NBK560707 [Last accessed on 2024 Apr 18]
- [Google Scholar]
- Antenatal diagnosis of total anomalous pulmonary venous connection in functional single ventricle hearts: Outcomes over 13-year period. Ultrasound. 2018;26:42-8.
- [CrossRef] [PubMed] [Google Scholar]
- Transposition of the great arteries with total anomalous pulmonary venous connection in a 1½ year-old child: Pulmonary arterial hypertension-An advantage. Ann Pediatr Cardiol. 2021;14:235-8.
- [CrossRef] [PubMed] [Google Scholar]
- Transposition of great arteries with total anomalous pulmonary venous connection: A modified Senning procedure for late presentation. JTCVS Tech. 2020;4:223-6.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal transient tricuspid valve regurgitation: Sonographic features and clinical evolution. J Matern Fetal Neonatal Med. 2021;34:2435-9.
- [CrossRef] [PubMed] [Google Scholar]







