Translate this page into:
Chronic sclerosing sialadenitis of the parotid gland mimicking a neoplasm
*Corresponding author: V. M. Mridhula, Barnard Institute of Radiology, Madras Medical College, Chennai, Tamil Nadu, India. mridhula180998@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sathyanathan B, Mridhula VM. Chronic sclerosing sialadenitis of the parotid gland mimicking a neoplasm. Case Rep Clin Radiol. doi: 10.25259/CRCR_97_2024
Abstract
Chronic sclerosing sialadenitis or Küttner tumor is a benign chronic inflammatory disease of the salivary gland, most commonly involving the submandibular salivary gland in middle aged adults. We describe the magnetic resonance imaging features of a rare case of Küttner tumor with isolated parotid gland involvement in a child, to emphasize on the need for increased awareness among radiologists regarding the possibility and imaging features of this condition, and to enable its timely diagnosis and appropriate management.
Keywords
Chronic sclerosing sialadenitis
Magnetic resonance imaging
Parotid gland lesion
INTRODUCTION
We report a rare case of chronic sclerosing sialadenitis (CSS), also known as Küttner tumor with isolated parotid gland involvement in a male child, emphasizing its magnetic resonance imaging (MRI) features and the need for increased awareness among radiologists and clinicians regarding the disease and its features for appropriate diagnosis and management.
CASE REPORT
A 12-year-old male patient presented with a painful swelling over the left jaw for 2 months. On examination, the swelling was smooth, firm, and tender. Fine-needle aspiration cytology of the swelling was reported as pleomorphic adenoma.
Pre-operative MRI of the neck was done using 3T MRI (Skyra Siemens Healthineers, Erlangen, Germany). Standard institute protocol was followed and signal acquisition was done using 20 channel head and neck coil. T1 axial, T2 axial and sagittal, short-tau inversion recovery (STIR) coronal, T1 fat-suppressed axial and dynamic contrast-enhanced imaging, axial diffusion-weighted imaging (DWI), and coronal gradient sequences were used. These revealed a relatively well-defined, lobulated lesion involving the superficial and deep lobes of the parotid gland, measuring approximately 1.9 × 1.9 × 2.6 cm. The lesion was hypointense on T1 images, predominantly T2 and STIR hypointense, with an irregular rim of hyperintensity and no signal loss on fat suppression. The surrounding parotid parenchyma appeared relatively T2 and STIR hyperintense than the contralateral parotid. Few areas within the lesion showed restricted diffusion on DWI with low apparent diffusion coefficient (ADC) values (0.96 × 10−3 mm2/s) and no foci of gradient blooming were observed [Figure 1]. Contrast enhancement was progressive and heterogenous, with time-signal intensity curve (TIC) showing gradual enhancement and no washout [Figure 2]. Few sub centimetric intraparotid lymph nodes were also observed. No similar lesion or abnormal hyperintensity was noted in the contralateral parotid gland, submandibular glands or elsewhere in the imaged field. Based on these features, possible differential diagnoses considered were solitary fibrous tumor, lymphoma, and CSS.
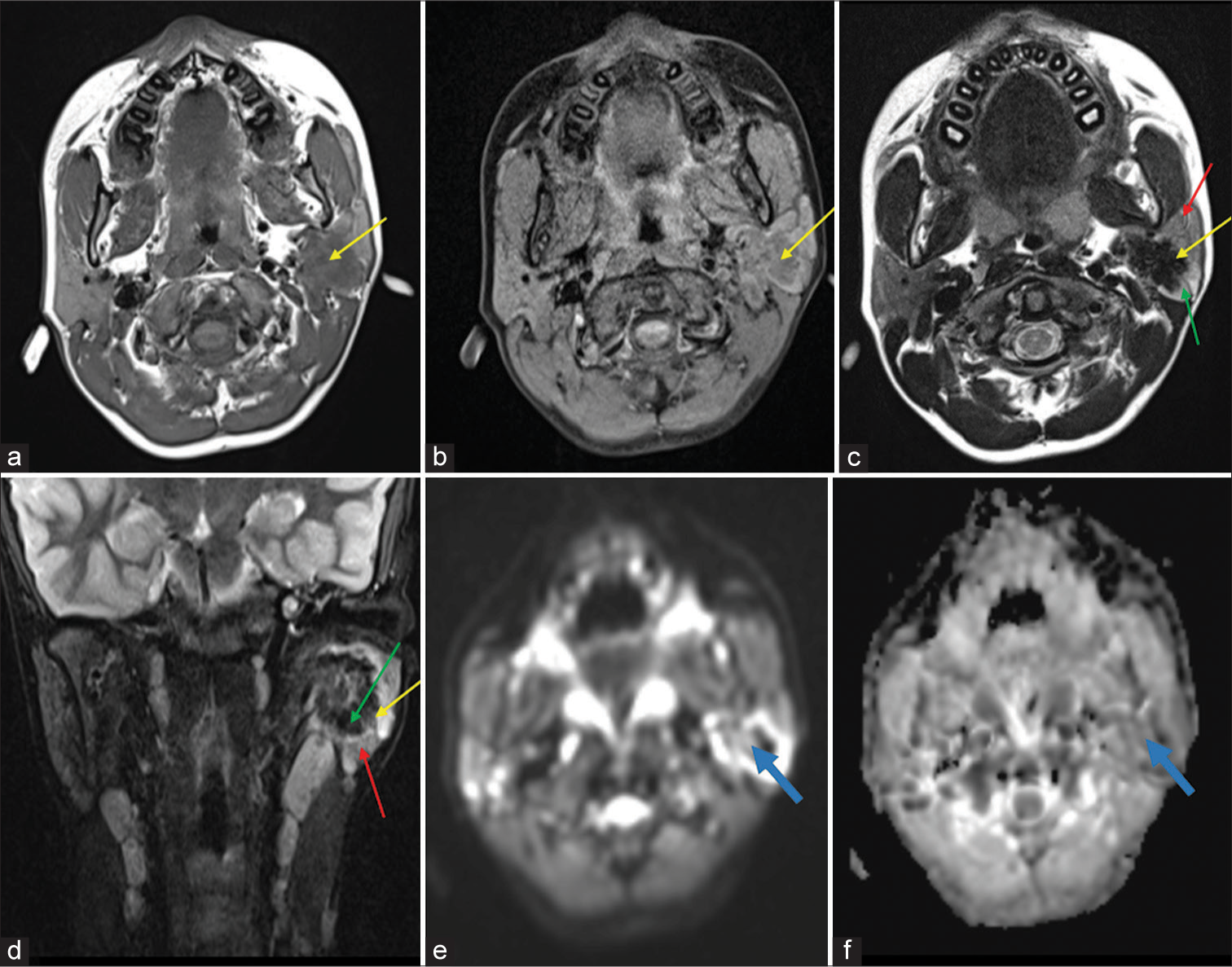
- (a) T1 axial image and (b) T1 fat-suppressed axial image showing a relatively well-defined, lobulated hypointense lesion (yellow arrows) in the left parotid gland. (c) Axial T2 and (d) Coronal short-tau inversion recovery images show that the lesion is predominantly hypointense (yellow arrows), with an irregular rim of hyperintensity (green arrows) and the surrounding parotid parenchyma appearing relatively hyperintense (red arrows) on comparison with the contralateral parotid. (e) Diffusion-weighted image and (f) Apparent diffusion coefficient map showing few areas of restricted diffusion in the lesion (blue arrows).
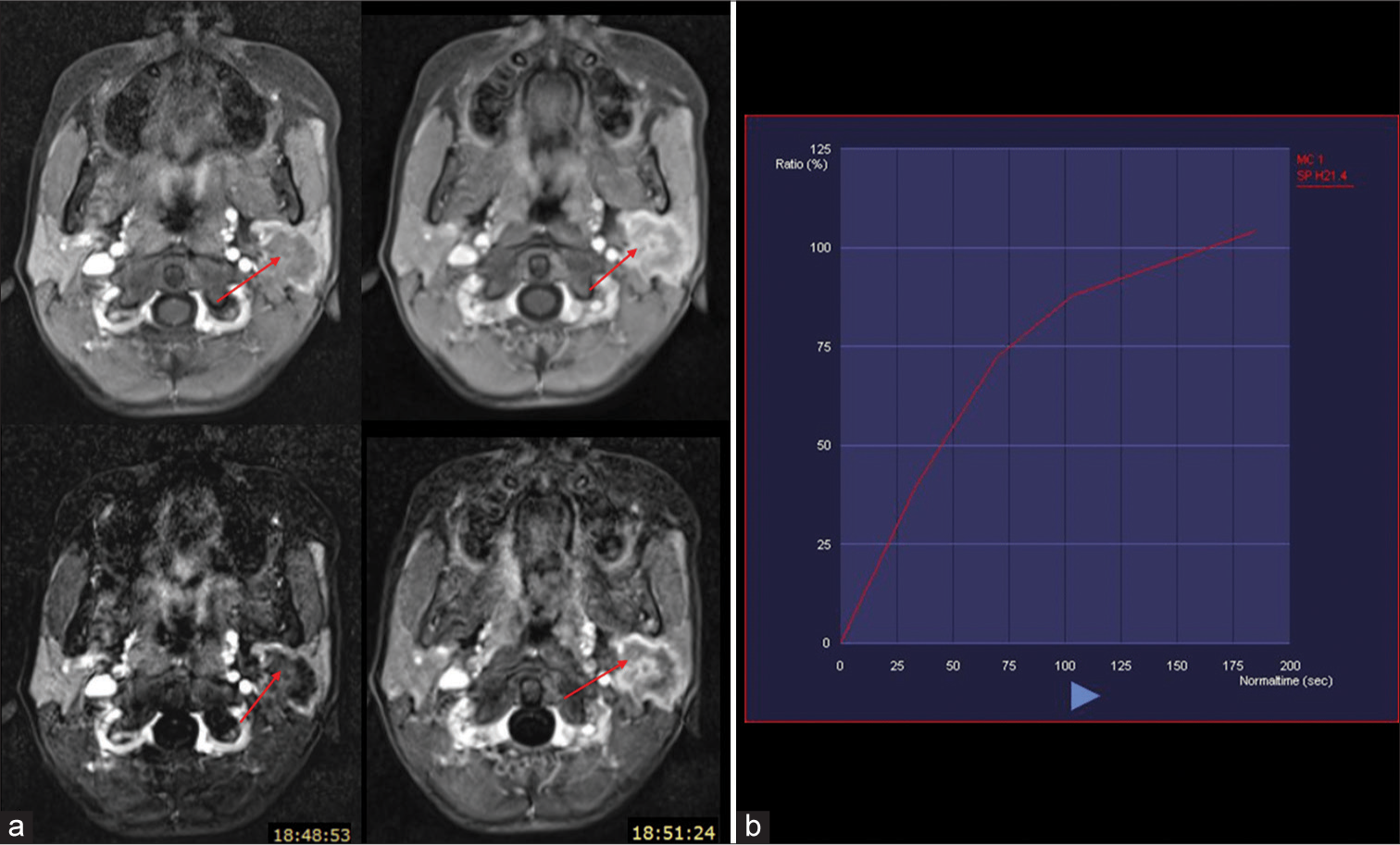
- (a) Dynamic contrast-enhanced (above) and subtracted (below) images showing progressive heterogenous enhancement of the lesion (red arrows). (b) Time-signal intensity curve showing gradual enhancement with no washout. Blue arrow signifies increasing time period from contrast administration.
The lesion was partially excised to avoid facial nerve injury. Histopathological examination (HPE) revealed dense fibrocollagenous stroma with periductal lymphoplasmacytic infiltrates forming lymphoid follicles [Figure 3], with reactive lymphadenitis of the intraparotid lymph node, establishing a diagnosis of CSS.
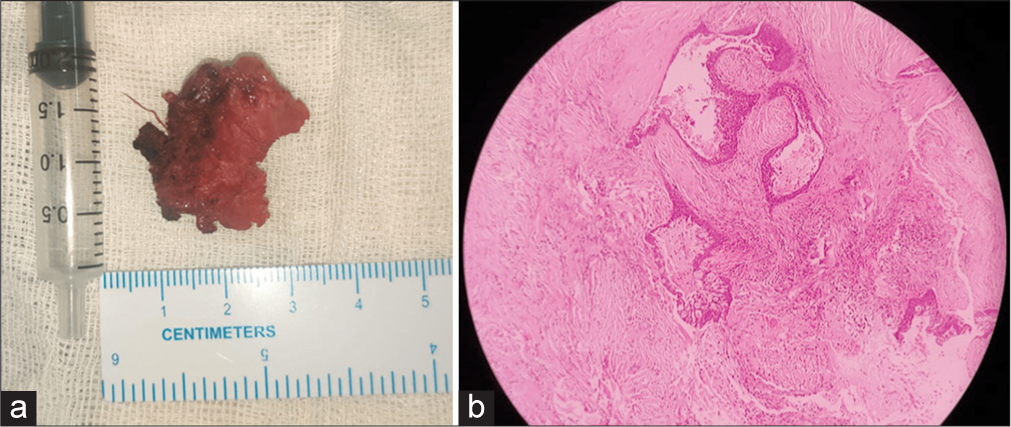
- (a) Gross specimen of the excised parotid gland specimen. (b) Histopathological examination shows dense fibrocollagenous stroma surrounding dilated ducts and periductal lymphoplasmacytic infiltrates.
The patient was discharged without further surgical intervention and is being managed conservatively.
DISCUSSION
CSS is a relatively uncommon, underreported benign chronic inflammatory disease of the salivary gland.[1] The condition is frequently encountered in the submandibular salivary gland unilaterally or bilaterally, or with multiglandular involvement including the submandibular, parotid, and sometimes lacrimal glands with cervical lymphadenitis.[2] Only four cases of isolated parotid gland involvement have been reported, with only one of them being pediatric and none with a detailed MRI description.[3] Studies attribute the etiopathogenesis to an immune reaction caused by an initial disturbance in secretion and obstructive electrolyte sialadenitis.[4] A strong association with immunoglobulin G4 (IgG4) disease[5] and sialolithiasis[4] have also been confirmed. In our case, there were no features of autoimmune condition or sialolithiasis. Histologically, Küttner’s tumor is characterized by lymphocytic infiltration around the salivary ducts progressing to diffuse, prominent infiltration, and follicle formation. In addition, there is also severe periductal fibrosis leading to the end stage parenchymal atrophy and sclerosis.[4] In our case as well, there was periductal lymphoplasmacytic infiltration and lymphoid follicles with dense sclerosis seen on HPE. CSS usually presents in middle aged adults as a firm, tender swelling with or without pain. The indurated consistency of the lesion commonly leads to misdiagnosis as neoplasm, leading to unnecessary sialadenectomy and surgical complications.[6] Our patient was pediatric, but the clinical features were similar. On MRI, submandibular gland lesions are ill defined,[1,7] while parotid lesions can be relatively well defined[3] or ill defined, especially in systemic IgG4 disease.[2] Signal intensities, diffusion restriction, and dynamic contrast enhancement pattern are determined by the proportion and degree of lymphocytic infiltration and fibrosis. Hence, the lesion is iso- to hypointense on T1 and usually hypointense on T2 and STIR images.[1,7] The gland surrounding the lesion shows relative T2 and STIR hyperintensity than the contralateral normal gland.[1] Restricted diffusion with low ADC values, similar to malignant tumors, is observed in the cellular rich areas of lymphocytic infiltration.[1,7] On dynamic contrast-enhanced MRI, the pattern is usually gradual and upward (type A TIC), due to the fibrotic component, as observed in our case. This can help in differentiating this entity from benign and malignant tumors which show rapid enhancement with early or delayed washout (type B, type C TIC). However, malignancy like salivary duct carcinoma with prominent fibrosis may show gradual enhancement while some cases of CSS can show rapid enhancement with or without washout. Therefore, enhancement pattern can support the diagnosis, but other morphological features are necessary to distinguish CSS from other entities strongly.[1,3,7,8] Metastatic lymphadenopathy which is commonly encountered in malignant tumors in usually not seen in CSS.[1,7] In our case, the MRI features were in accordance with these features.
Various differential diagnoses can be considered while analyzing a case of CSS [Table 1]. A differential diagnosis of solitary fibrous tumor had been considered as these lesions are of intermediate signal intensity on T1 and variable signal intensity on T2-weighted images, but the attribute of intense homogenous contrast enhancement seen in solitary fibrous tumor was absent in our case.[9] Lymphoma, another possible differential diagnosis, is characterized by hypointensity on T1-weighted images with low- to high-signal intensity on T2-weighted images and low, homogenous contrast enhancement.[10] The lesion in our case had similar signals, but showed heterogenous, progressive enhancement. Further, there were no cystic changes or calculi, which are commonly seen in parotid lymphoma. While the lesion in our case had relatively well-defined, lobulated margins, and a type A TIC on dynamic contrast, both of which are common in pleomorphic adenoma, other supporting features such as T2 hyperintensity with a hypointense rim and absence of significantly restricted diffusion were not seen.[11] Chronic infective sialadenitis in adults as well as pediatric patients is characterized by enlargement of the gland with mixed T2 signal intensity and microcystic areas due to duct ectasia.[12] Inflammatory pseudotumor is another rare differential diagnosis, which shows T1 and T2 hypointensity with homogenous or peripheral enhancement and relatively higher ADC values (>1.25 × 10−3 mm2/s).[13] Kimura disease commonly involves the parotid glands and presents as a mass lesion. However, on MRI, it is characteristically hyperintense on T1- and T2-weighted images with internal flow voids and moderate to marked contrast enhancement.[14]
While some studies advocate surgical resection due to risk of malignant transformation,[7] few suggest that correct diagnosis and medical management directed at the immune nature of the disease can avoid unnecessary sialadenectomy and surgical complications.[2] In our case too, partial excision was done and not sialadenectomy, avoiding facial nerve injury and following the histopathological diagnosis, no further surgical intervention was considered.
DIFFERENTIAL DIAGNOSIS
Imaging features that help distinguish other differential diagnoses from chronic sclerosing sialadenitis are given below [Table 1].
| Differential diagnosis | Differentiating features |
|---|---|
| 1. Solitary fibrous tumor | • Intermediate T1 signal intensity • Variable T2 signal intensity • Intense homogenous contrast enhancement |
| 2. Lymphoma | • T1 hypointensity • Variable T2 signal intensity • Homogenous contrast enhancement • Significantly low ADC values • Cystic areas, calculi |
| 3. Malignant tumor | • Unclear, invasive margins • Displacement of adjacent blood vessels • Metastatic cervical lymphadenopathy |
| 4. Pleomorphic adenoma | • T2 hyperintensity with hypointense rim • No diffusion restriction |
| 5. Chronic infective sialadenitis | • Mixed T2 signal intensity • Microcystic areas due to duct ectasia |
| 6. Inflammatory pseudotumor | • Mild to moderate homogenous or peripheral enhancement • ADC value>1.25×10−3 mm2/s |
| 7. Kimura disease | • T1, T2 hyperintense • Flow voids • Moderate to marked enhancement |
ADC: Apparent diffusion coefficient.
CONCLUSION
CSS with isolated parotid gland involvement has rarely been reported and in our review of literature, no case detailing the MRI features of a parotid Küttner tumor in a pediatric patient could be found till date. Awareness regarding the possibility of this entity as well as its imaging features is crucial for timely confirmation of its benign nature and appropriate management, avoiding unnecessary surgery and complications.
TEACHING POINTS
CSS mimics neoplasm both clinically and imaging wise and is prone for misdiagnosis, thereby leading to unnecessary sialadenectomy and surgical complications.
MRI features of this disease may help to distinguish it from other differential diagnoses.
MCQs
-
Chronic sclerosing sialadenitis most commonly involves the
Parotid gland
Submandibular gland
Sublingual gland
Lacrimal gland
Answer key: b
-
Clinical features of chronic sclerosing sialadenitis usually include:
Firm consistency
Fever
Painful swelling
Predilection for middle aged adults
Answer key: a, c, d
-
All of the following are features of chronic sclerosing sialadenitis except:
T2 hypointensity
Low ADC values
Rapid contrast enhancement with washout
T2 hyperintensity of the surrounding parenchyma
Answer key: c
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- MRI of chronic sclerosing sialoadenitis. Br J Radiol. 2008;81:531-6.
- [CrossRef] [PubMed] [Google Scholar]
- IgG4-related systemic disease affecting the parotid and submandibular glands: Magnetic resonance imaging features of IgG4-related chronic sclerosing sialadenitis and concomitant lymphadenitis. Clin Imaging. 2014;38:195-8.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic sclerosing sialadenitis (Küttner's tumour) of the parotid gland. Malays J Med Sci. 2010;17:57-61.
- [Google Scholar]
- On the pathogenesis of the Kuttner tumour of the submandibular gland: analysis of 349 cases with chronic sialadenitis of the submandibular gland. HNO. 1977;25:81-92.
- [Google Scholar]
- Chronic sclerosing sialadenitis (Küttner tumor) is an IgG4-associated disease. Am J Surg Pathol. 2010;34:202-10.
- [CrossRef] [PubMed] [Google Scholar]
- 'Kuttner's tumour (chronic sclerosing sialadenitis) of the submandibular gland: A clinical perspective. Hong Kong Med J. 2008;14:46-9.
- [Google Scholar]
- MRI features in submandibular gland chronic sclerosing sialadenitis: A report of three cases and imaging findings. Iran J Otorhinolaryngol. 2020;32:397-401.
- [Google Scholar]
- Salivary gland tumors: Diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology. 2003;226:345-54.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of solitary fibrous tumors in the head and neck. Korean J Radiol. 2005;6:136-42.
- [CrossRef] [PubMed] [Google Scholar]
- Non-Hodgkin lymphoma involving the parotid gland: CT and MR imaging findings. Dentomaxillofac Radiol. 2013;42:20130046.
- [CrossRef] [PubMed] [Google Scholar]
- MRI criteria for the diagnosis of pleomorphic adenoma: A validation study. Am J Otolaryngol. 2014;35:713-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nasopharyngeal inflammatory pseudotumor: Multimodality imaging characterization. Eur J Radiol Extra. 2009;72:e61-4.
- [CrossRef] [Google Scholar]
- Kimura disease: CT and MR imaging findings. AJNR Am J Neuroradiol. 2012;33:784-8.
- [CrossRef] [PubMed] [Google Scholar]








