Translate this page into:
Wolf in sheep’s clothing
*Corresponding author: J. Jayaranjeetham, Department of Radiodiagnosis, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. jayranji94@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jayaranjeetham J, Ramachandran M, Sunitha VC. Wolf in sheep’s clothing. Case Rep Clin Radiol. doi: 10.25259/CRCR_22_2024
Abstract
Autoimmune encephalitis (AE) is an immune-mediated disease with a varied clinical spectrum. Osmotic demyelination is a condition characterized by hypernatremia following an episode of correction for hyponatremia. Our case report is about a patient who presented with features of central diabetes insipidus and an imaging diagnosis of osmotic demyelination. Magnetic resonance imaging of the brain, which later turned out to be AE based on the positive auto-antibodies.
Keywords
Autoimmune encephalitis
Magnetic resonance imaging
Central diabetes insipidus
Osmotic demyelination
INTRODUCTION
Autoimmune encephalitis (AE) is an important diagnostic consideration in patients with a new onset of altered mental status with no obvious etiology.[1] Due to its variety of clinical presentations and its mimicking nature of various pathological process, it poses a diagnostic challenge to clinicians.[1] AE is a diagnosis of exclusion.[2,3] Antibodies against voltage-gated potassium channel (VGKC) or ligand-gated N-methyl-d-aspartate receptor (NMDAR), glycine receptor (GlyR) ion channels, or ungated water aquaporin 4 (AQP4) channels are some of the antibodies that have been found in AE.[4] Based on the presence or absence of the underlying malignancy, auto-immune disorders can be divided into paraneoplastic and non-neoplastic types.[5-7] Another classification of AE is (i) AE with antibodies against intracellular antigens (Hu, CV2, Amphiphysin, Ri, Yo, Ma2 or Ta, GAD) and (ii) AE with antibodies against surface antigens (N-methyl-d-aspartate [NMDA], VGKC [including ligand gated ion channel (LGI-1), contactin-associated protein-like 2 (CASPR-2), and contactin-2], Gamma-aminobutyric acid-b (GABA-b), α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), Glycine, mGLuR1, Voltage gated calcium channel [VGCC]).[8]
Findings in magnetic resonance imaging (MRI) include multiple and confluent T2/fluid attenuated inversion recovery (FLAIR) hyperintense areas, showing gadolinium enhancement and no obvious diffusion restriction, involving the cortical and subcortical areas of bilateral cerebral hemispheres.[9] The mesial temporal lobe and limbic systems are commonly involved. The involvement of the basal ganglia is also frequent, which is the imaging feature differentiating AE from herpes simplex encephalitis.[10]
Osmotic demyelination is a condition caused by raised sodium levels following a rapid correction of hyponatremia.[11] Extrapontine myelinosis affects the bilateral capsulo-ganglionic region, corpus callosum, hippocampus, lateral geniculate body, thalami, cerebellum, subependymal region, spinal cord, and other territories of the cerebral cortex.[12,13] It rarely occurs in acute hypernatremia.[12]
CASE REPORT
A 24-year-old female presented to our institute with a history of progressive weakness of all 4 limbs for 1 week and fever, loose stools, burning micturition, seizure, slurring of speech, and altered sensorium for 2 days. She also gave a history of excessive water intake and an increased frequency of micturition for 2 months. She was treated for gastroenteritis at a peripheral hospital 1 week before visiting our institute. Her sodium levels while she was admitted to the peripheral hospital were high 165 mEq/L, and at the time of discharge, they were 148 mEq/L.
Cerebrospinal fluid (CSF) analysis done in our institute showed no cells, a normal protein level, and a glucose level 70% of that of the serum level. Liver function tests and renal function tests were within normal limits. A sample was also sent for auto–antibody workup.
Based on history, clinical features, and biochemical investigation reports, a diagnosis of central diabetes insipidus was made. She was started on desmopressin, and hypernatremia was corrected. Meanwhile, she underwent an MRI of her brain, which showed symmetrical T2/FLAIR hyperintensities in the bilateral basal ganglia, bilateral external capsule, splenium of the corpus callosum, and subcortical white matter hyperintensities in the left temporal and right frontal lobes. On diffusion-weighted imaging, these areas showed restricted diffusion. Susceptibility-weighted imaging showed no blooming foci [Figure 1].
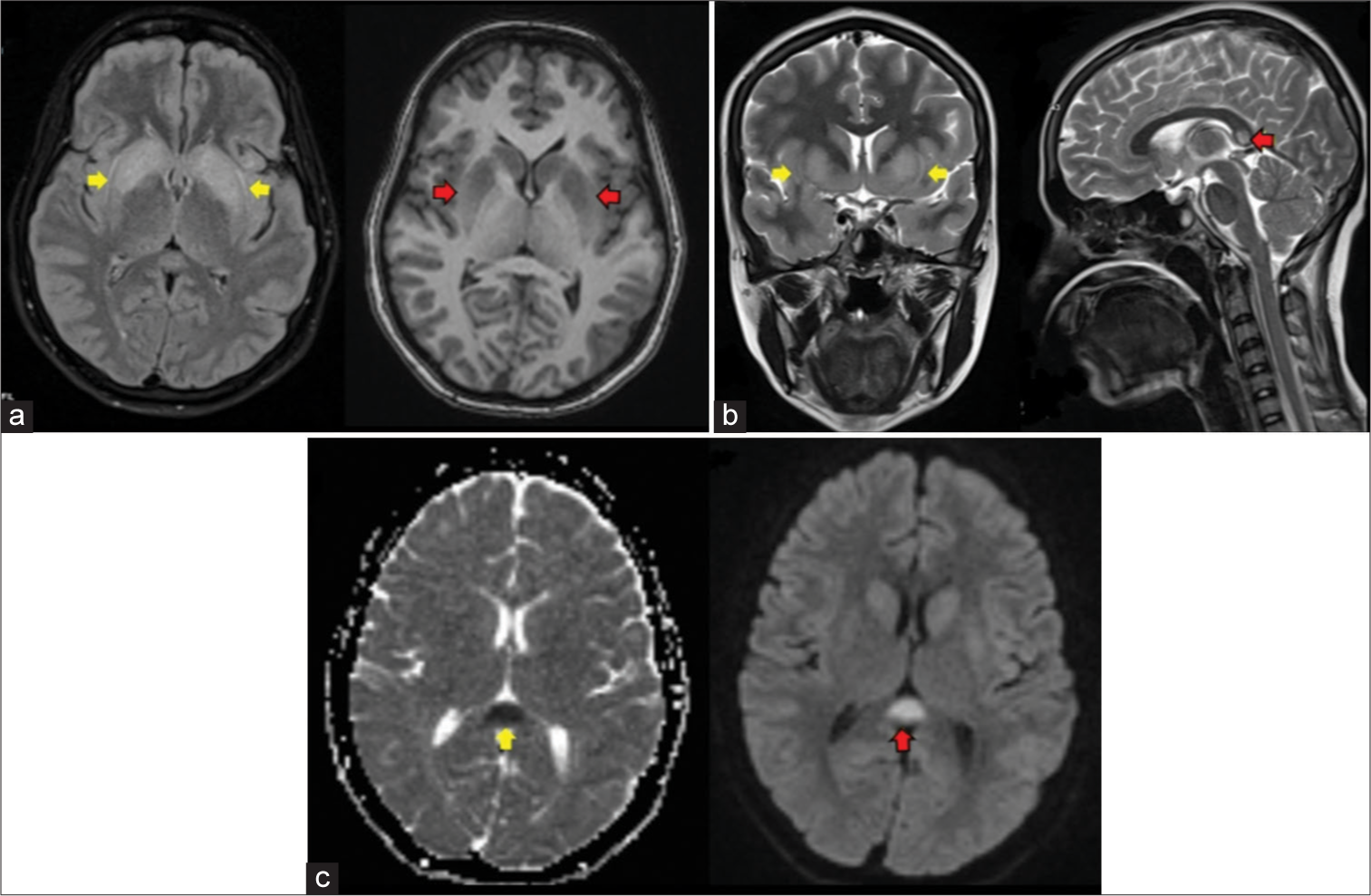
- A 24-year-old woman with autoimmune encephalitis who presented with altered sensorium (a) FLAIR-axial shows hyperintensity in bilateral basal ganglia (yellow arrows), T1-axial shows hypointensity in bilateral basal ganglia (red arrows) (b) T2-coronal shows hyperintensity in bilateral basal ganglia (yellow arrows), T2-sagittal shows hyperintensity in splenium of corpus callosum (red arrow), (c) Apparent diffusion coefficient (ADC)-axial shows hypointensity in splenium of corpus callosum (yellow arrow), Diffusion weighted imaging (DWI)-axial shows hyperintensity in splenium of corpus callosum (red arrow).
Correlating the history of hypernatremia, clinical features, and imaging findings, the possibility of osmotic demyelination was considered. The patient was suggested follow-up imaging on the recovery of symptoms. She was treated symptomatically.
After 2 weeks, a repeat MRI of the brain was done, showing mild resolution of the hyperintensities in the bilateral capsulo–ganglionic region and a significant reduction of the hyperintensity in the splenium of the corpus callosum, as shown in Figure 2. This was used for confirmation of the osmotic demyelination.
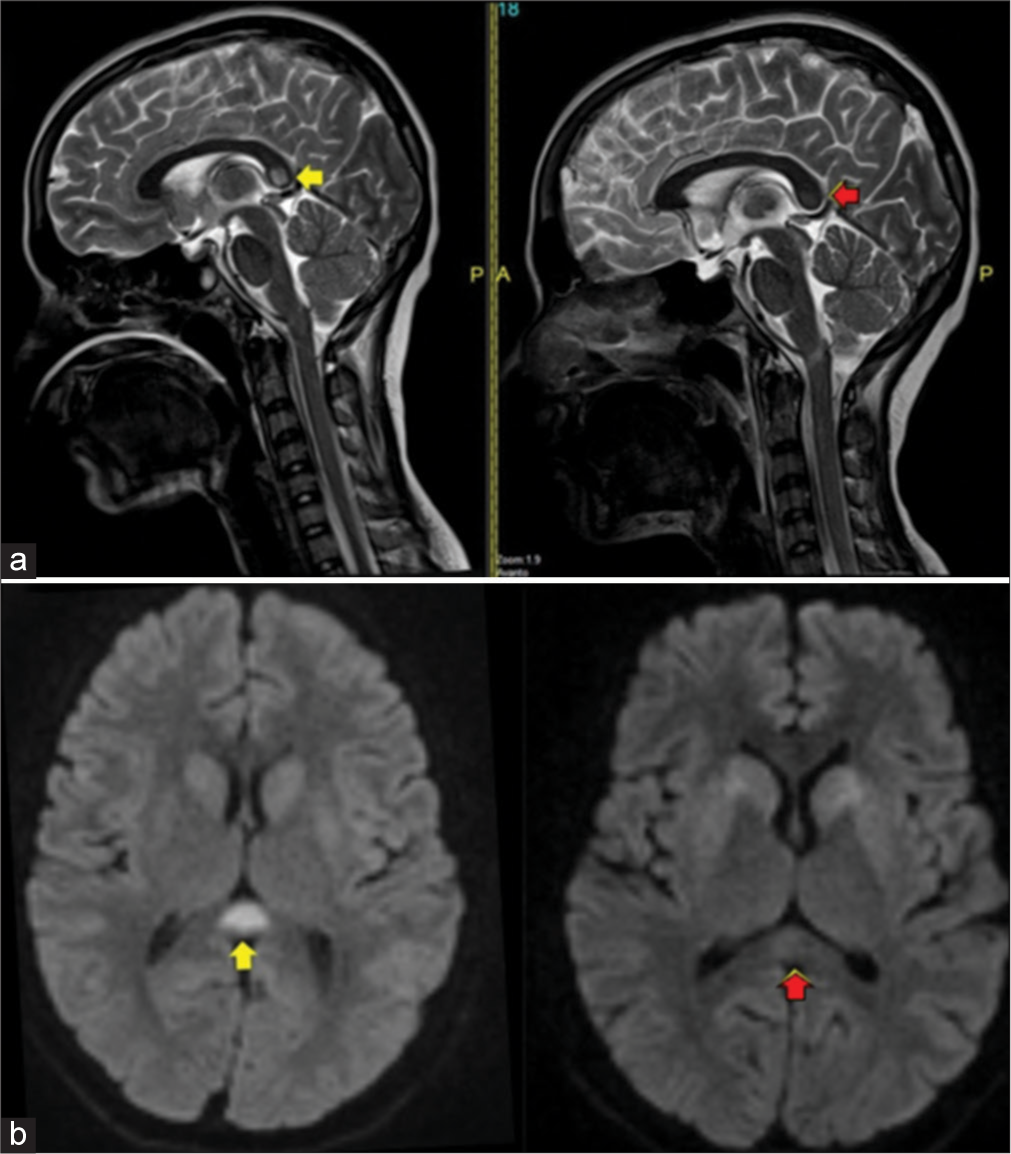
- A 24-year-old woman with autoimmune encephalitis who presented with altered sensorium (a) T2-sagittal shows hyperintensity in splenium of corpus callosum at presentation (yellow arrow), T2-sagittal shows significant decrease of hyperintensity after 2 weeks (red arrow) (b) DWI-axial shows restricted diffusion in splenium of corpus callosum at presentation (yellow arrow), DWI-axial shows significant decrease of restricted diffusion after 2 weeks (red arrow).
Meanwhile, the patient got the results of the antibody panel test (CSF) done in the peripheral hospital, which turned out to be positive for anti-NMDA, and based on this, a final diagnosis of AE was made, and she was started on IV Ig for 5 days. Following treatment, she showed improvement in sensorium in the form of increased awareness and improvement in the power of the limbs. On further inquiry, the patient provided a history of recurrent abortions.
DISCUSSION
Osmotic demyelination is a condition that occurs commonly in chronic alcoholics and also follows the rapid correction of hyponatremia. Our patient had a history of gastroenteritis, was treated for the same outside, and presented with hypernatremia, possibly due to dehydration. Pons is the part most commonly involved in central pontine myelinosis. The MRI of our patient showed features of extra–pontine myelinosis in the form of T2 hyperintensities in bilateral capsule-ganglionic region, splenium of the corpus callosum. Hence, the patient was started on symptomatic treatment for the same. A repeat MRI after 2 weeks showed resolution of the hyperintensity in the splenium of the corpus callosum. Mild resolution of the signal changes in the capsule-ganglionic region also confirmed our diagnosis though the patient did not show complete improvement. Once the results of the antibody panel were obtained, she was re-diagnosed with autoimmune encephalitis.
Autoimmune encephalitis is a diagnosis of exclusion. There are various mimics of auto-immune encephalitis, which include tumors, infection, and demyelination.
Autoimmune encephalitis can be classified as paraneoplastic or non-paraneoplastic based on the presence or absence of the malignancy. Other classifications are based on antibodies. In paraneoplastic encephalitis, autoantibodies are directed against intracellular antigens, while in non-paraneoplastic encephalitis, antibodies are directed against surface antigens. Examples of various antibodies are anti-HU, anti-NMDA, anti-Ma, anti-Ta, anti-GAD, anti-Ro, anti-Yo, anti-GABA, etc. NMDAr AE is classically seen in young women and children. Our patient is a young female with a recurrent history of abortions. Now that she was diagnosed with AE with a positive antibody test, the recurrent abortions could be attributed to the antiphospholipid antibody syndrome (APLA) syndrome.
Early diagnosis and treatment of NMDAr encephalitis have a good prognosis. On follow up of the patient, there is complete regression and disappearance of imaging findings. Most of the patients with NMDAr encephalitis will have normal MRI findings. In the presence of imaging findings, there is wide variation in the distribution and degree of T2/FLAIR hyperintense signal changes throughout the brain.
Overall, imaging findings in AE indicate involvement of the limbic system. Other areas known to be involved in AE are the striatum, diencephalon, rhombencephalon, spine, and peripheral nervous system. The lateral temporal lobe and insula are less commonly involved. This is the finding that differentiates autoimmune encephalitis from herpes simplex encephalitis. The imaging findings in our patient are similar to these.
For the diagnosis of autoimmune encephalitis, not only imaging findings but also the clinical spectrum, serum and CSF–antibody panel are also required. After the patient was diagnosed with auto–immune encephalitis, she was started on IV IG, and she showed rapid improvement.
Thus, autoimmune encephalitis is a wolf in sheep’s clothing that may present in various forms, mimicking various conditions. The radiologist must be aware of the various presentations of the AE and should raise the possibility of diagnosis or include it as a differential diagnosis whenever required.
DIFFERENTIAL DIAGNOSIS
Osmotic demyelination
Metabolic encephalopathy
Herpes encephalitis.
CONCLUSION
It is important to the know the varied manifestations of autoimmune encephalitis and should raise a strong suspicion especially when the patient does not respond to the empirical treatment. As early diagnosis and treatment would greatly benefit the patient.
TEACHING POINTS
AE is a diagnosis of exclusion and may present in various forms, mimicking various imaging features
The clinical spectrum, laboratory findings, and imaging findings may aid in diagnosing AE
Extrapontine myelinosis is a close mimicker of AE.
MCQs
-
Extrapontine myelinosis involves
Lateral geniculate body
Spinal cord
Thalami
All of the above
Answer Key: d
-
Which of the following statements regarding NMDAr encephalitis is true?
Has a poor prognosis
Most commonly seen in the elderly population
Most of the time, will have normal imaging findings
None of the above.
Answer Key: c
Acknowledgment
We would like to acknowledge the kind help provided by the Department of Medicine.
Ethical approval
Institutional review board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Autoimmune encephalitis: Pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38:1070-8.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging of rapidly progressive dementias, part 1: Neurodegenerative etiologies. AJNR Am J Neuroradiol. 2014;35:418-23.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging of rapidly progressive dementias, part 2: Prion, inflammatory, neoplastic, and other etiologies. AJNR Am J Neuroradiol. 2014;35:424-31.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune channelopathies: New antibody-mediated disorders of the central nervous system. F1000 Biol Rep. 2009;1:61.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune encephalitis in the ICU: Analysis of phenotypes, serologic findings, and outcomes. Neurocrit Care. 2016;24:240-50.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63-74.
- [CrossRef] [PubMed] [Google Scholar]
- Paraneoplastic limbic encephalitis: Neurological symptoms, immunological findings and tumour association in 50 patients. Brain J Neurol. 2000;123(Pt 7):1481-94.
- [CrossRef] [PubMed] [Google Scholar]
- Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257:509-17.
- [CrossRef] [PubMed] [Google Scholar]
- MRI characteristics of autoimmune encephalitis with autoantibodies to GABAA receptor: A case series. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1158.
- [CrossRef] [PubMed] [Google Scholar]
- Limbic encephalitis: A clinical-radiological comparison between herpetic and autoimmune etiologies. Eur J Neurol. 2013;20:1566-70.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid correction of hyponatremia causes demyelination: Relation to central pontine myelinolysis. Science. 1981;211:1068-70.
- [CrossRef] [PubMed] [Google Scholar]
- A case of osmotic demyelination presenting with severe hypernatremia. Electrolyte Blood Press. 2015;13:30-6.
- [CrossRef] [PubMed] [Google Scholar]
- Central pontine and extrapontine myelinolysis: The great Masquerader-an autopsy case report. Case Rep Neurol Med. 2014;2014:745347.
- [CrossRef] [PubMed] [Google Scholar]








